Articles
Article Tools
Stats or Metrics
Article
Original Research
Exp Neurobiol 2010; 19(1): 39-48
Published online June 30, 2010
https://doi.org/10.5607/en.2010.19.1.39
© The Korean Society for Brain and Neural Sciences
Normal Adult Hippocampal Neurogenesis in SRG3-overexpressing Transgenic Mice
Byungwoo Kim1, Eugene Lee1, Rho H. Seong2, Heekyoung Chung3 and Hyeon Son1*
Departments of 1Biochemistry and Molecular Biology and 3Pathology, Hanyang University College of Medicine, Seoul 133-791, 2Department of Biological Sciences, Institute of Molecular Biology and Genetics and Research Center for Functional Cellulomics, Seoul National University, Seoul 151-742, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-2220-0626, FAX: 82-2-2294-6270
e-mail: hyeonson@hanyang.ac.kr
SRG3 (SWI3-related gene) is a core subunit of mouse SWI/SNF complex and is known to play a critical role in stabilizing the SWI/SNF complex by attenuating its proteasomal degradation. SWI/SNF chromatin remodeling complex is reported to act as an important endogenous regulator in the proliferation and differentiation of mammalian neural stem cells. Because limited expression of SRG3 occurs in the brain and thymus during mouse embryogenesis, it was hypothesized that the altered SRG3 expression level might affect the process of adult hippocampal neurogenesis. Due to the embryonic lethality of homozygous knockout mice, this study focuses on dissecting the effect of overexpressed SRG3 on adult hippocampal neurogenesis. The BrdU incorporation assay, immunostaing with neuronal markers for each differentiation stage, and imunoblotting analysis with intracellular molecules involved in survival in adult hippocampal neurogenesis found no alteration, suggesting that the overexpression of SRG3 protein in mature neurons had no effect on the entire process of adult hippocampal neurogenesis including proliferation, differentiation, and survival.
Keywords: SRG3 (SWI3-related gene), SWI/SNF complx, adult hippocampal neurogenesis, proliferation, differentiation, survival
INTRODUCTION
Mammalian SWI/SNF complex, also known as BAF (Brg/Brm-associated factor) complex, is a >2 MDa multiprotein chromatin remodeling complex composed of at least 10 elements and contain BRG or BRM subunit as a central core ATPase subunit. All subunits of SWI/SNF are well conserved from yeast to human, reflecting the functional significance of the SWI/SNF complex (Tsukiyama, 2002). SWI/SNF complex is involved in various biological events such as tumor suppression, regulation of immune system, neural development and plasticity (Roberts and Orkin, 2004). A diverse composition of SWI/SNF complex subunits determines which transcription factors or coactivators/corepressors activate or repress target genes. For example, transcription factors including C/EBP, c-Myc and c-Fos/c-Jun activate target genes by cooperating with the SWI/SNF complex (Cheng et al., 1999). Likewise, the SWI/SNF complex represses neuronspecific genes in nonneuronal cells through the association with neuron restrictive silencer factor (NRSF) and its corepressors, mSin3A and CoREST (Battaglioli et al., 2002; Watanabe et al., 2006).
SRG3 (SWI3-related gene) is a recently found subunit of mouse SWI/SNF complex and has a high homology to a yeast transcriptional activator SWI3 and human BAF155 proteins (Jeon et al., 1997). Although SRG3 is widely expressed during early mouse embryogenesis, its main expression is limited to thymus and brain around E18.5, suggesting a role for SRG3 in the immune and the nervous system (Kim et al., 2001). SRG3 heterozygote mice frequently show exencephaly (Kim et al., 2001). Interestingly, the expression pattern of SRG3 overlaps with that of Brg1, and Brg1 heterozygote mice also have high incidence of exencephaly (Bultman et al., 2000). Brg1 is constantly expressed during differentiation of neural progenitor cells (NPC) and P19 embryonic carcinoma cells (Machida et al., 2001). It is also required for the proneural activites of Ngnr1, NeuroD and for neuronal differentiation in both
The whole step of adult neurogenesis is subdivided into proliferation, cell cycle exit, differentiation and functional maturity (Ehninger and Kempermann, 2008). Each step is tightly controlled by a variety of extrinsic and intrinsic factors, most of which remain uncharacterized (Zhao et al., 2008). Intriguingly, SWI/SNF chromatin remodeling complex acts as an important endogenous regulator in the proliferation and differentiation of mammalian neural stem cells (Lessard et al., 2007). The switch of subunits from BAF45a and BAF53a to BAF45b, BAF45c, and BAF53b is essential for the transition from neural stem/progenitors to postmitotic neurons, suggesting that the genetic disturbance of only one subunit might disorganize the entire process of adult hippocampal neurogenesis. Therefore, in this study, the functional significance of SRG3 and how chromatin remodeling complex contributes to maintain the integrity of adult hippocampal neurogenesis was approached by analyzing the effect of overexpressed SRG3 on adult hippocampal neurognesis.
MATERIALS AND METHODS
The knock-in transgenic (TG) mice overexpressing SRG3 were provided by Dr. R.H. Seong (Seoul National University). These mice are heterozygous for overexpressed SRG3 and produced and maintained in FVB background. The mouse SRG3 gene is designed to be overexpressed in neurons of forebrain regions by mouse CaMKII (calcium/calmodulin-dependent protein kinase II) α promoter (11.5 kb) (Benson et al., 1992; Jacobs et al., 1993; Mayford et al., 1996). The transgenic offspring was generated by crossing heterozygous transgenic mice with wild type FVB mice and genotyped by PCR using sense primer 5'-GACTA GACCA AACAT CTACC TC-3', antisense primer 5'-GTCAA CTGAG CGACT TGGAT C-3'. Mice were bred and maintained under pathogen-free conditions, and experiments were performed in accordance with institutional guidelines of Hanyang University Veterinary Committee. To evaluate the proliferation in subgranular zone (SGZ) of adult hippocampus, 5-bromo-2'-deoxyuridine (BrdU) (100 mg/kg bodyweight, Sigma-Aldrich) was daily administered intraperitoneally to twenty week-old male TG mice for 3 days and then animals were sacrificed 2 h after the last injection.
Immunoblotting followed conventional method. Briefly, hippocampal and cortical tissues were immediately collected from an isolated brain on ice after mice were sacrificed by cervical translocation. Tissues were homogenized using a Dounce homogenizer and lysed in RIPA buffer (150 mM NaCl, 10 mM Tris, 0.1% SDS, 1% Triton X-100, 5 mM EDTA, 1% Deoxycholate) including protease inhibitor cocktail (Sigma-Aldrich)/phosphatase inhibitor cocktail (Sigma-Aldrich). The kinds and dilutions of primary antibodies are as follows; anti-BAF155 (1:500, Santa Cruz Biotechnology Inc), anti-phospho-CaMKII (Thr286) (1:1,000; Cell Signaling Technology), anti-CaMKII (1:500, Cell Signaling Technology), anti-β-actin (GeneTex), anti-p-Trk (1:400, Cell Signaling Technology), anti-TrkB (1:600, Cell Signaling Technology), anti-p-ERK1/2 (1:1,000, Cell Signaling Technology), anti-ERK1/2 (1:1,000, Cell signaling Technology), anti-p-CREB (Ser133) (Abcam Inc, Cambridge, MA, USA), anti-CREB (1:500, Cell Signaling Technology), anti-BDNF (1:500, SantaCruz Biotechnology), anti-VEGF (1:200, SantaCruz Biotechnology), anti-Angiopoietin-1 (1:200, SantaCruz Biotechnology), anti-Tuj1 (1:1,000, Covance, Berkeley, CA, USA), anti-Brg1 (1:1,000, Sigma-Aldrich).
Sample tissues were collected in the same way as mentioned in immunoblotting and homogenized in Trizol reagent (Life Technologies Inc, Rockville, MD, USA). One µg of total RNA was reversetranscribed using SuperScript II reverse transcriptase (Invitrogen). The following PCR primers were employed; sense 5'-CTGCATCTTCGATCACC TCA-3', antisense 5'-CTCCCTTCCAGCACTAGCAC-3' for mouse SRG3; sense 5'-CGGAACCGC TCATTGCC-3', antisense 5'-ACCCACACTGTGCCCATCTA-3' for β-actin sense.
After anesthetized with Ketamine/Xylazine cocktail, animals were intracardially perfused with cold normal saline and, in turn, ice-cold 4% paraformaldehyde in PBS. Isolated brains were postfixed in 4% paraformaldehyde in PBS overnight at 4℃, and then fully dehydrated in 30% sucrose in PBS. Slices were prepared as 30-µm-thick. For immunoperoxidase staining, free-floating sections were first treated with 1% H2O2 for 15 min and washed three times with PBS. After incubated in blocking buffer (0.3% Triton X-100/5% normal serum in PBS) for 1 h, they were placed in primary antibody solution overnight at 4℃. Primary antibodies used are goat anti-BAF155 antibody (1:200, Santa Cruz Biotechnology), anti-p-CREB (Ser133) (1:200, Abcam Inc, Cambridge, MA, USA), and anti-BDNF (1:500, SantaCruz Biotechnology). Biotinylated anti-goat IgG antibody (Vector Laboratories, Burlingame, CA, USA) was used as a secondary antibody. Immunoreactivity was detected by Vectastain ABC Kit (Vector Laboratories) and DAB peroxidase substate kit (Vector Laboratories). For BrdU immunofluorescent staining, sections were incubated in 50% formamide/50% 2X SSC buffer (0.3 M NaCl and 0.03 M sodium citrate) at 65℃ for 2 h and rinsed with 2X SSC buffer for 10 min. They were then incubated for 30 min in 2 N HCl at 37℃ and rinsed in 0.1 M boric acid (pH 8.5) for 15 min. After PBS wash, they were incubated in blocking buffer for 1 h and rat anti-BrdU antibody (1:200, Abcam) solution overnight at 4℃. For Tuj-1 (1:400, Covance), PSA-NCAM (1:100, Chemicon International), NeuN (1:500, Chemicon International), MAP2 (1:400, Sigma-Aldrich) and Dcx (1:200, Santa Cruz Biotechnology), the incubation in formamide/2X SSC buffer and 2 N HCl was skipped. Cy3-conjugated anti-IgG (Jackson ImmunoResearch, West Grove, PA, USA) and Alexa Fluor 488-conjugated anti-IgG (Molecular Probes, Eugene, OR, USA) were used as secondary antibody. The samples were mounted with Vectashield (Vector Laboratories).
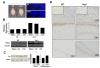
To confirm whether SRG3 overexpression transgenic mice are working as genetically designed to be driven by CaMKIIα promoter, which expresses in postmitotic neurons in forebrain regions like neocortex, hippocampus and amygdala (Benson et al., 1992; Jacobs et al., 1993), SRG3 TG mice were examined to see forebrain-specific overexpression of SRG3. The gross brain morphology and size of 20-week-old male SRG3 TG was not different from that of wild type (Fig. 1A), suggesting that overall development of brain is not affected by the overexpression of SRG3. Both hemispheres were symmetrical, and the size of cerebellum and olfactory bulb where CaMKIIα promoter is not activated also looks similar in SRG3 TG and wild type (wt) animals. The normal development of neocortex and the hippocampal subregions like dentate gyrus (DG), CA3 and CA1 of SRG3 TG mouse was confirmed by DAPI labeling on a coronal section at postbregma -2.0 mm (Fig. 1A). The expression of SRG3 was evaluated at the levels of mRNA and protein. Real-time RT-PCR showed the forebrain specific overexpression of SRG3 gene (Fig. 1B, hippocampus, 2.6-fold increase; cortex, 2.4-fold increase; cerebellum, 1.3-fold increase; respectively compared with wt, n=1). The SRG3 protein level revealed by western blotting using antiserum against BAF155, a human homologue of SRG3, also significantly increased in SRG3 TG mice compared to in wild type (mean±SEM; hippocampus, 143.7±5% vs wild; cortex, 137.9±5.6% vs wild, *p<0.05 respectively, n=3 per group) (Fig. 1C). In order to compare the expression lelvel of SRG3 protein in individual neurons, immunoperoxidase acativity against SRG3 was examined in brain slices from both groups. The SRG3 signal was detected in the nuclei (Fig. 1D inset) and it displayed more intense signals in SRG3 TG mice forebrain region than in wt (Fig. 1D). These results consistently demonstrate that the genetic manipulation for a SRG3 overexpression TG mouse is reliablely working, showing an increased expression pattern for SRG3 mRNA and protein in forebrain neurons.
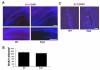
To determine whether the overexpression of SRG3 causes an altered pattern in the proliferation of neural progenitor cells, the number of newly generated progenitor cells in SGZ was examined by the incorporation of BrdU. Although CaMKIIα promoter does not operate in proliferating cells, the neural progenitor cells in the DG of adult hippocampus can be affected indirectly by postmitotic neurons (Chen et al., 2007) overexpressing SRG3 gene by CaMKIIα promoter. BrdU immunopositive (BrdU+) cells were counted within SGZ throughout the DG of both genotypes (Fig. 2A). Some BrdU+ cells were found in clusters like those in the previous reports (Yagita et al., 2001; Zhu et al., 2005; Hodge et al., 2008). The total number of BrdU+ cells located in the SGZ (mean±SEM; wt, 1,099±63/mm3 vs. SRG3 TG, 1,024±59/mm3, p>0.1, n=4 per group) was not statistically different between wt and SRG3 TG mice (Fig. 2B), indicating that the proliferation was not affected by the overexpression of SRG3.
Cell proliferation in the subventricular zone (SVZ) of a lateral ventricle was also examined (Gould, 2007). Though CaMKIIα promoter operates only in matured neurons, a possible effect of the overexpression of SRG3 on the proliferation pattern of SVZ was needed to be checked when considering of SVZ as another important niche for adult neurogenesis. However, no difference in the proliferation rate in SVZ was found in both genotypes (Fig. 2C).
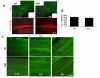
To determine whether early neuronal development is normally organized in SRG3 TG mice, postomitotic differentiation in a hippocampal region was immunostained with early neuronal markers. During postmitotic and neuronal differentiation stage in adult hippocampal neurogenesis, most newborn neurons are destined to die, but only survived neurons mature to integrate into a functional neural circuit (Tashiro et al., 2007). Previous studies reported that cells committed to a neuronal lineage start to express doublecortin (Dcx) (Francis et al., 1999) and the polysialylated embryonic form of the neural cell adhesion molecule (PSA-NCAM), right after they exit out of the cell cycle (Fukuda et al., 2003). The total number of Dcx immunoreactive (Dcx+) cells in the hippocami of SRG3 TG mice was not significantly changed compared to that of wt (mean number±SEM: wt, 9,974±450/mm3; SRG3 TG, 9,133±701/mm3, p>0.1, n=4 per group), suggesting that neuronal differentiation of neural progenitor cells in the hippocampus of adult SRG3 TG mice seems normal during early postmitotic period (Fig. 3A). As Dcx expression is temporally overlapped with PSA-NCAM expression on the time course of development, the immunoreactivity of PSA-NCAM+ cells on randomly chosen brain slices from both genotypes cells was compared (Nacher et al., 2001). It turns out that the number and the signal intensity of PSA-NCAM+ cells was apparently similar to result of Dcx immunostaining and there is no difference between SRG3 TG and wt groups.
In order to know what effect the overexpressed SRG3 on mature neurons has on the maturation of the hippocampi and cortex, Tuj-1 was used as a neuroblast marker as well as a fully differentiated neuronal marker to examine SRG3 TG mice. Neuron-specific class III β-tubulin (Tuj-1) has been found to be expressed in newly generated immature postmitotic neurons (Menezes and Luskin, 1994) and in mature neurons (Ambrogini et al., 2004) (Fig. 3C). Tuj-1 was detected in somata and neurites of neurons within cortex, CA1 and DG. Especially, in DG, Tuj-1 was detected in the somata of almost all granule cells and found at the neurites of some granule cells reaching the hippocampal fissure. The distribution and immunoreactivity of Tuj-1 in cortex, CA1 and DG was indistinguishable between SRG3 TG and wt animals, suggesting that the neuronal maturation in adult hippocampal neurogenesis as well as cortical neuronal development appear normal in SRG3 TG mice.
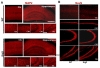
To see whether overexpressed SRG3 affects the late neuronal differentiation process and the survival of neuronal lineage-committed cells during the late stage of adult hippocampal neurogenesis, microtubule-associated protein 2 (MAP2) and NeuN were immunolabeled, as MAP2 and NeuN have been known as mature neuronal markers (Menezes and Luskin, 1994). The signal was detected at dendrites and somata of neurons (Fig. 4A) as reported previously (Biranowska et al., 2000; Jalava et al., 2007). Though there were some cells in SGZ missing MAP2 immunoreactivity, they might be immature or proliferating cells. The distribution and immunoreactivity of MAP2 immunopositive (MAP2+) cells in the cortex and the hippocampus was not significantly different in SRG3 TG and wt animals (Fig. 4A). In addition, the immubolabeling of NeuN, a nuclear protein for labeling the nuclei of mature neurons (Lind et al., 2005), displayed a similar pattern in SRG3 TG and wt mice (Fig. 4B), suggesting that the late neuronal differentiation process during adult hippocampal neurogenesis and the neuronal survival remains normal in SRG3 TG mice.
Though the immunostaining with MAP2 and Tuj-1 results in no impaired pattern of the survival of newly generated hippocampal neurons by the overexpressed SRG3, this might not mean that the neuronal maturation is completely normal. To determine whether the neuronal survival is not altered in a subtle manner by the overexpression of SRG3, CREB activation, which is a critical step for survival of newborn neurons in a hippocampus (Nakagawa et al., 2002), was examined using western blotting and immunolabeling. ERK1/2 and CaMKII, critical mediators in activating CREB, were expressed and activated to a similar degree (Fig. 5A, pERK1/2, 104±6% vs wt, p>0.05; pCaMKII, 97±2% vs wt, p>0.05), and similarly CREB activation was not different between two groups (Fig. 5A, pCREB, 106±3% vs wt, p>0.05). Moreover, the expression of BDNF regulated by phosphorylated CREB and the activation of TrkB, a receptor for BDNF, were not significantly different in both groups (Fig. 5A: BDNF, 103±3% vs. wt, p>0.05; pTrkB, 106±5% vs wt, p>0.05), which is consistent with the immunolabeling of each marker on tissues, implying that neuronal survival and the survival signaling is not affected by the overexpression of SRG3 in mature neurons.
To see whether the expression of VEGF and angiopoietin1 was altered by the overexpressed SRG3, the western blotting was performed with using hippocampal homogenates from each genotype, because vascular endothelial growth factor (VEGF) and angiopoietin1 are all angiogenesisrelated and were reported to be linked to adult neurogenesis (During and Cao, 2006; Warner-Schmidt et al., 2008). Recently, SRG3 has been reported to be required for angiogenesis and visceral endoderm development in the yolk sac, and the expression of angiogenesis-related genes, includeing Angiopoietin1, Tie2, EphrinB2, Ihh and Notch1, was markedly reduced in SRG3-/-Tg+ yolk sacs (Han et al., 2008). The expression of VEGF and angiopoietin1 was not different in both genotypes (Fig. 5A: VEGF, 104±3% vs wt, p>0.05; angiopoietin1, 102±3% vs wt, p>0.05), unlike the reduced expression of angiogenesis related genes by the depletion of SRG3.
To overcome the possible limitation of the western blotting missing the subtle change of the altered expression level of specific molecules which are very small portion of the whole hippocampus, the activation or expression of BDNF and CREB was examined by immunofluorescent and immunoperoxidase staining at a single cell level (Fig. 5B, C). BDNF signal was not strongly detected at somata and dendritic arbors of hippocampal neurons from both groups and rather more intensely observed in neurites of cortical neurons (Fig. 5B). The phosphorylated CREB was detected in nuclei of cells within SGZ and across cortical layers (Fig. 6C). Taken together, a line of evidence from western blotting and immunostaining with phosphorylated-CREB and BDNF clearly demonstrate that overexpression of SRG3 makes no significant impact on the expression and activation of intracellular signaling molecules involved in the survival of newborn neurons in adult hippocampus.
TG mice overexpressing SRG3 by CaMKIIα promoter only in neurons of forebrain regions such as hippocampus were used to explore the role of SRG3 protein, a core subunit of SWI/SNF chromatin remodeling complex, in adult hippocampal neurogenesis
BrdU incorporation assay demonstrated no alteration in the proliferation within DG of SRG3 TG mice. This is consistenly supported by the western blotting analysis showing that there is no difference in the expression level of neurogenic factors such as VEGF and BDNF. SRG3 is overexpressed specifically in postmitotic neurons so that its direct influence on proliferating cells within hippocampus might not be accessed. However, this might not be simply unchanged pattern caused by SRG3 overexpression. The transient increase in the number of neuronal cells in DG of SRG3 TG mice might be compensated by subsequently increaseed cell death and, as a consequence, the total number of early neuronal marker-positive cells went down to a normal level. However, TUNEL assay showed that cell death level was also not changed by overexpressed SRG3 (data not shown), and gross brain morphology and DAPI-coronal section staining showed neither brain atrophy nor neuronal loss, indicating there is no abnormal ratio in cell death in SRG3 TG mice.
With respect to early neuronal differentiation during adult hippocampal neurogenesis, the total numbers and expression patterns of Dcx, PSA-NCAM and Tuj-1 molecules were similar between wt and SRG3 TG mice. This might be because the expression of early neuronal marker proteins such as Dcx, PSA-NCAM precedes the CaMKIIα expression (Plumpe et al., 2006; Earnheart et al., 2007). Immunolabeling with MAP2 and NeuN, late neuronal differentiation markers, also showed no difference between wt and SRG3 TG mice even though NeuN+ or MAP2+ cells would overexpress SRG3 protein by the control of CaMKIIα promoter. It might imply that SRG3 overexpression in mature neurons do not necessarily affect the expression of late differentiation markers. In this point, the downregulated level of SRG3 might answer the issue how SRG3 might contribute to adult hippocampal neurogenesis.
It may be argued that it was an improper choice for studying the role of SRG3 in newly generated neurons, because CaMKIIα promoter operates only in postmitotic cells committed to neurons. However, some reports that neural stem/progenitor cells communicate with neighboring cells like endothelial cells, microglia or astrocytes in a neurogenic niche (Ma et al., 2005) and that neurotrophic or angiogenic factors act in a paracrine manner (Emanueli et al., 2003) suggest a possibility that the overexpression of SRG3 might affect the generation of neurons in adult hippocampus. Therefore, it might be premature to conclude that SRG3 overexpression has no significant effect on adult hippocampal neurogenesis. Rather, it might need an electrophysiological approach or exploration at the synaptic level to detect subtle change of structural plasticity possibly induced by the overexpression of SRG3.
In summary, the present study demonstrates that overexpressed SRG3 in hippocampal neurons has no influence on the entire process of adult hippocampal neurogenesis including proliferation, differentiation, and survival
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morphofunctional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res 2004;1017:21-31.
- Battaglioli E, Andres ME, Rose DW, Chenoweth JG, Rosenfeld MG, Anderson ME, Mandel G. REST repression of neuronal genes requires components of the hSWI.SNF complex. J Biol Chem 2002;277:41038-41045.
- Benson DL, Isackson PJ, Gall CM, Jones EG. Contrasting patterns in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience 1992;46:825-849.
- Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell 2000;6:1287-1295.
- Chen K, Henry RA, Hughes SM, Connor B. Creating a neurogenic environment: the role of BDNF and FGF2. Mol Cell Neurosci 2007;36:108-120.
- Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol 2003;258:319-333.
- Cheng SW, Davies KP, Yung E, Beltran RJ, Yu J, Kalpana GV. c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function. Nat Genet 1999;22:102-105.
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res 2006;3:29-33.
- Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res 2008;331:243-250.
- Emanueli C, Schratzberger P, Kirchmair R, Madeddu P. Paracrine control of vascularization and neurogenesis by neurotrophins. Br J Pharmacol 2003;140:614-619.
- Eroglu B, Wang G, Tu N, Sun X, Mivechi NF. Critical role of Brg1 member of the SWI/SNF chromatin remodeling complex during neurogenesis and neural crest induction in zebrafish. Dev Dyn 2006;235:2722-2735.
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 1999;23:247-256.
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestinpositive cells in adult mouse dentate gyrus. J Neurosci 2003;23:9357-9366.
- Gould E. How widespread is adult neurogenesis in mammals?. Nat Rev Neurosci 2007;8:481-488.
- Han D, Jeon S, Sohn DH, Lee C, Ahn S, Kim WK, Chung H, Seong RH. SRG3, a core component of mouse SWI/SNF complex, is essential for extra-embryonic vascular development. Dev Biol 2008;315:136-146.
- Jacobs KM, Neve RL, Donoghue JP. Neocortex and hippocampus contain distinct distributions of calciumcalmodulin protein kinase II and GAP43 mRNA. J Comp Neurol 1993;336:151-160.
- Jalava NS, Lopez-Picon FR, Kukko-Lukjanov TK, Holopainen IE. Changes in microtubule-associated protein-2 (MAP2) expression during development and after status epilepticus in the immature rat hippocampus. Int J Dev Neurosci 2007;25:121-131.
- Jeon SH, Kang MG, Kim YH, Jin YH, Lee C, Chung HY, Kwon H, Park SD, Seong RH. A new mouse gene, SRG3, related to the SWI3 of Saccharomyces cerevisiae, is required for apoptosis induced by glucocorticoids in a thymoma cell line. J Exp Med 1997;185:1827-1836.
- Kim JK, Huh SO, Choi H, Lee KS, Shin D, Lee C, Nam JS, Kim H, Chung H, Lee HW, Park SD. SRG3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol 2001;21:7787-7795.
- Lessard J, Wu JI, Ranish JA, Wan M, Winslow MM, Staahl BT, Wu H, Aebersold R, Graef IA, Crabtree GR. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron 2007;55:201-215.
- Lind D, Franken S, Kappler J, Jankowski J, Schilling K. Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. J Neurosci Res 2005;79:295-302.
- Ma DK, Ming GL, Song H. Glial influences on neural stem cell development:cellular niches for adult neurogenesis. Curr Opin Neurobiol 2005;15:514-520.
- Machida Y, Murai K, Miyake K, Iijima S. Expression of chromatin remodeling factors during neural differentiation. J Biochem 2001;129:43-49.
- Menezes JR, Luskin MB. Expression of neuronspecific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci 1994;14:5399-5416.
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci 2001;14:629-644.
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci 2002;22:3673-3682.
- Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Romer B, Rodriguez GR, Kronenberg G, Kempermann G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci 2006;7:77.
- Roberts CW, Orkin SH. The SWI/SNF complex-chromatin and cancer. Nat Rev Cancer 2004;4:133-142.
- Seki T, Arai Y. Temporal and spacial relationships between PSA-NCAM-expressing, newly generated granule cells and radial glia-like cells in the adult dentate gyrus. J Comp Neurol 1999;410:503-513.
- Seo S, Richardson GA, Kroll KL. The SWI/SNF chromatin remodeling protein Brg1 is required for vertebrate neurogenesis and mediates transactivation of Ngn and NeuroD. Development 2005;132:105-115.
- Sohn DH, Lee KY, Lee C, Oh J, Chung H, Jeon SH, Seong RH. SRG3 interacts directly with the major components of the SWI/SNF chromatin remodeling complex and protects them from proteasomal degradation. J Biol Chem 2007;282:10614-10624.
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci 2007;27:3252-3259.
- Tsukiyama T. The in vivo functions of ATP-dependent chromatin-remodelling factors. Nat Rev Mol Cell Biol 2002;3:422-429.
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia 2006;54:815-825.
- Watanabe H, Mizutani T, Haraguchi T, Yamamichi N, Minoguchi S, Yamamichi-Nishina M, Mori N, Kameda T, Sugiyama T, Iba H. SWI/SNF complex is essential for NRSF-mediated suppression of neuronal genes in human nonsmall cell lung carcinoma cell lines. Oncogene 2006;25:470-479.
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008;132:645-660.
- Zhu H, Dahlstrom A, Hansson HA. Characterization of cell proliferation in the adult dentate under normal conditions and after kainate induced seizures using ribonucleotide reductase and BrdU. Brain Res 2005;1036:7-17.