Articles
Article Tools
Stats or Metrics
Article
Review Article
Exp Neurobiol 2013; 22(3): 143-148
Published online September 30, 2013
https://doi.org/10.5607/en.2013.22.3.143
© The Korean Society for Brain and Neural Sciences
Brain-Region Specific Apoptosis Triggered by Eph/ephrin Signaling
Soochul Park*
Department of Biological Science, Sookmyung Women's University, Seoul 140-742, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-710-9330, FAX: 82-2-715-9331
e-mail: scpark@sookmyung.ac.kr
Abstract
- Go to
- Abstract
- INTRODUCTION
- EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- PERSPECTIVES
- Figure
- Reference
Eph receptors and their ligands, ephrins, are abundantly expressed in neuroepithelial cells of the early embryonic brain. Overstimulation of Eph signaling
Keywords: EphA, ephrin-A, apoptosis, early brain development
INTRODUCTION
- Go to
- Abstract
- INTRODUCTION
- EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- PERSPECTIVES
- Figure
- Reference
The cerebral neocortex is unique to mammals, and its size varies among species [1]. The cerebral cortex appears complicated and proportionately the largest in humans. It would be quite interesting to understand the molecular mechanisms underlying the various sizes of mammalian brains. A balance between cell survival and death might be a key determinant modulating the size of mammalian brains. For example, the early elimination of neuroepithelial cells in the embryonic brain has been reported to be a critical mechanism of normal brain development [2-4]. One class of molecules implicated in the early elimination of neuroepithelial cells is comprised of Eph receptors and their ligands, ephrins. This review will focus on the recent advances in understanding the potential role of Eph/ephrin signaling in inducing brain region-specific apoptosis.
EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- Go to
- Abstract
- INTRODUCTION
- EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- PERSPECTIVES
- Figure
- Reference
Eph receptors are grouped into two different subclasses based on their amino acid similarities and ligand binding preferences [5, 6]. EphA receptors bind to ephrin-As, which are tethered to cell membrane through glycophosphatidylinositol (GPI) anchors. EphB receptors bind to ephrin-Bs, which have their own transmembrane domain and short cytoplasmic region. Therefore, an important feature of Eph receptors and ephrin ligands is that they are capable of transmitting bi-directional signaling at sites of cell-cell contact: Eph receptors mediate forward signaling and ephrin ligands mediate reverse signaling. However, some reports indicate that certain Eph receptors and ephrin ligands have more complex binding preferences than expected [7, 8]. For example, ephrin-A5 binds to all EphA subtype receptors but also it binds to EphB2, a member of EphB subtype receptors [9]. However, the biological significance of this unusual interaction between ephrin-A5 and EphB2 remains to be determined
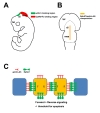
Eph receptors and their ligands are abundantly expressed in the early developing brain [7]. Interestingly, Eph receptors and their corresponding ligands have mutually exclusive expression domains. This observation has primarily resulted from in situ staining of Eph receptor-Fc or ephrin ligand-Fc fusion proteins. For example, ephrinA5-Fc protein binds to EphA receptors in the dorsal part of the diencephalon and anterior mesencephalon, whereas EphA8-Fc protein binds to ephrin-A ligands in the dorsal region of the posterior mesencephalon (Fig. 1A). Therefore, it seems that Eph receptors and their corresponding ligands encounter each other only at interfaces between their expression domains. This reciprocal expression pattern of Eph receptors and ephrin ligands has been suggested as a key mechanism for organizing the body plan during development. Consistently, it has been shown that Eph-expressing cells are effectively sorted out of ephrin-expressing cells through a cell-cell repulsive mechanism. Thus, mutually exclusive expression patterns of Eph and ephrin may be an efficient mechanism allowing Eph- and ephrin-expressing cells to segregate from each other, thereby sharpening the boundary between Eph- and ephrin-expressing cells.
EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- Go to
- Abstract
- INTRODUCTION
- EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- PERSPECTIVES
- Figure
- Reference
In situ staining of Eph and ephrin fusion proteins is a powerful technique for observing overall expression patterns of Eph receptors and ephrin ligands in the developing embryo [7]. However, this technique is not sensitive enough to detect regions where Eph receptors and ephrin ligands are co-expressed. Eph and ephrin fusion proteins do not effectively detect these regions because Eph receptors are masked by pre-bound ephrin ligands, or vice versa, at sites of cell-cell contact. One example is the nasal region of the retina where ephrin-As are co-expressed with EphA receptors [10]. The masking of EphA receptors in nasal retinal ganglion cell axons may contribute to their appropriate targeting to the posterior part of the superior colliculus. In this interesting system, EphA receptors are pre-bound to ephrin-A ligands through cis-interactions within single retinal ganglion cells, and this masking of Eph receptors allows retinal ganglion cell axon to be desensitized and therefore not repelled from ephrinA-expressing superior colliculus cells.
However, in spinal motor axon growth cones, EphA receptors and ephrin-A ligands are co-expressed but laterally segregated into distinct membrane domains [11]. Furthermore, EphA receptors on the growth cone mediate its collapse, whereas ephrin-As on the growth cone have the opposing effects of axon growth and attraction. This study strongly suggests that Eph receptors and their corresponding ligands are arranged in trans-configuration within a single cell. More importantly, this subcellular localization of Eph receptors and ephrin ligands allow them to act as distinct receptors within the same neuronal cell. Therefore, co-expression of Eph and ephrin is likely to play a physiologically relevant role during brain development, and it is important to identify relevant brain regions using more sophisticated techniques, such as the expression of reporters specifically inserted into a gene locus.
ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- Go to
- Abstract
- INTRODUCTION
- EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- PERSPECTIVES
- Figure
- Reference
The first clue that Eph/ephrin signaling triggers apoptotic cell death came from a study in which ephrin-A5 was ectopically over-expressed in EphA7-expressing neuroepithelial cells [12]. In this study, a full-length human ephrin-A5 cDNA was targeted into the first exon of EphA7 bacterial artificial chromosome (BAC), and its ectopic expression was induced by Emx1-Cre, which is specifically expressed in telecephalic neuroepithelial cells. Using this strategy, EphA7-expressing cells were selectively induced to express ephrin-A5, resulting in severe apoptotic cell death and a dramatic decrease in cortical size. This finding suggests that co-expression of Eph and ephrin in neuroepithelial cells may be an important mechanism inducing apoptotic cell death and reducing the size of their population. Consistent with this finding, EphA7 null mutant embryos exhibited excencephalic overgrowth of the forebrain, suggesting that neuroepithelial cells in the forebrain undergo less apoptotic cell death in the absence of EphA7. However, the ectopic expression of ephrin-A5 in EphA7-expressing cells could exert artificial effects on EphA7, with over-expressed ephrin-A5 possibly in cis-configuration with EphA7 within the same cell. This unnatural cis-complex of EphA7/ephrin-A5 might desensitize EphA7 and inhibit cross-talk with other cell surface receptors that are critical for cell survival. Therefore, the strategic approach that ephrin-A5 is ectopically expressed in the neuroepithelial cells expressing endogenous ephrin-A5 would be desirable for a gain-of-function study of Eph/ephrin signaling during early brain development.
EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- Go to
- Abstract
- INTRODUCTION
- EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- PERSPECTIVES
- Figure
- Reference
The dorsal midline of the diencephalon and mesencephalon was shown to be a region where EphA7 and ephrin-A5 are co-expressed [13]. This study revealed that ephrin-A5 is co-expressed with EphA7 at the edges of neural folds prior to their fusion. It was further postulated that truncated EphA7 receptors block repulsive interactions between full-length EphA7 and ephrin-A5 and that this process is essential for the fusion of neural folds during embryonic development. Importantly, ephrin-A5 null mutant embryos displayed neural tube defects caused by the failure of neural folds to fuse at the midline. However, the neural tube defect observed in ephrin-A5 knock-out embryos appears to depend on the genetic background of null mutant mice. For example, neural tube closure defects were hardly observed among ephrin-A5 null mutant embryos in a C57BL/6J background. Also, it remains to be determined whether a truncated version of EphA7 lacking a tyrosine kinase domain plays a physiologically relevant role
A recent study in which a LacZ reporter was inserted into the ephrin-A5 and EphA7 BAC more clearly demonstrates that EphA7 and ephrin-A5 are co-expressed in the dorsal midline of the diencephalon and mesencephalon at embryonic day (E) 10.5~12.5 (Fig. 1B). In this dorsal midline region, naturally occurring apoptotic cell death is frequently observed and is implicated in neural tube closure [14]. In chicks, the inhibition of apoptotic cell death in the dorsal midline region impairs efficient neural tube closure, resulting in an exencephalic or anencephalic phenotype. Also, genetically mutant mice lacking both JNK1 and JNK2 show less apoptotic cell death at the neural fold edges and a defect in neural tube closure [15]. In ephrin-A5 null mutant embryos, the number of EphA7-expressing cells is significantly increased in the dorsal midline of the diencephalon and mesencephalon [16]. These studies strongly suggest that, in the dorsal midline region of the diencephalon and mesencephalon, neuroepithelial cells transmit robust EphA7/ephrin-A5 signaling at sites of cell-cell contact, and this bi-directional signaling may have a biochemical linkage with the pro-apoptotic pathway (Fig. 1C). During fusion of the dorsal neural folds, cell-cell contact may be strong enough to strengthen the interaction between EphA7 and ephrin-A5, resulting in elimination of a subset of cells in this region. Even after neural tube closure, it is possible that transient up-regulation of either EphA7 or ephrin-A5 may be linked with pro-apoptotic signaling, and this mechanism may regulate the size of the neuroepithelial cell population in the dorsal midline region of the diencephalon and mesencephalon.
EphrinA5-Fc, a soluble form of membrane-tethered ephrin-A5, has been widely used to stimulate EphA receptors on the cell surface [13]. Although preclustering ephrinA5-Fc with anti-Fc secondary antibodies enhances its stimulatory effect on EphA receptors, ephrinA5-Fc fusion protein also has a stimulatory effect on EphA receptors without its preclustering [16]. Therefore, it is predicted that ephrinA5-Fc stimulates forward signaling through EphA receptors when its expression is induced in cells expressing both EphA7 and ephrin-A5. Does
What about
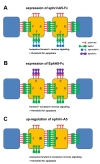
Based on findings of massive apoptotic cell death in neuroepithelial cells expressing ephrinA5-Fc or EphA8-Fc, the following hypotheses are proposed. First, in a brain region where EphA and ephrin-A are co-expressed, cell-cell contact induces strong bi-directional signaling downstream of the Eph/ephrin complex. However, this signaling may remain below a threshold that triggers pro-apoptotic cell death (Fig. 1C). Second, when activation of either reverse or forward signaling occurs in addition to the pre-existing bi-directional Eph/ephrin signaling at sites of cell-cell contact, the total signaling may be above a threshold for activation of the pro-apoptotic pathway (Fig. 2A, B). In this respect, it is possible that a transient wave of up-regulation of either ephrin or Eph gene expression might play a causative role in triggering excessive Eph/ephrin signaling, which is linked with activation of the pro-apoptotic signaling pathway. This aberrant regulation of ephrin or Eph gene expression may also provide a plausible mechanism for controlling the size of the neuroepithelial cell population and remodeling brain tissue (Fig. 2C).
PERSPECTIVES
- Go to
- Abstract
- INTRODUCTION
- EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- PERSPECTIVES
- Figure
- Reference
How does bi-directional signaling of the Eph/ephrin complex activate the pro-apoptotic pathway? As a recent study suggested, one possible scenario is that EphA receptors may crosstalk with cell death receptors such as TNFR1, although their interaction in vivo remains to be determined [18]. An alternative hypothesis is that EphA receptors crosstalk with cell survival receptors such as Trk. Upon ligand binding, this receptor crosstalk may be disturbed, resulting in greater activation of the pro-apoptotic pathway. On the other hand, reverse signaling through ephrin-A type ligands requires co-receptors such as RET tyrosine kinase receptor [19] or p75NTR [20], which might be disturbed or stimulated upon binding of EphA receptors. More intensive research regarding the apoptotic roles of receptor tyrosine kinases, cell death receptors, or caspases in bi-directional signaling of the Eph/ephrin complex would facilitate the understanding of brain-region specific apoptosis and brain tissue remodeling at the molecular level.
Figures
- Go to
- Abstract
- INTRODUCTION
- EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- PERSPECTIVES
- Figure
- Reference
References
- Go to
- Abstract
- INTRODUCTION
- EPH RECEPTORS AND THEIR CORRESPONDING LIGANDS ARE RECIPROCALLY EXPRESSED DURING EARLY EMBRYOGENESIS
- EPH RECEPTORS ARE CO-EXPRESSED WITH THEIR CORRESPONDING LIGANDS IN CERTAIN BRAIN REGIONS
- ECTOPIC EXPRESSION OF EPHRIN-A5 RESULTS IN APOPTOTIC CELL DEATH OF EPHA7-EXPRESSING NEUROEPITHELIAL CELLS
- EXPRESSION OF EPHRINA5-FC AND EPHA8-FC RESULTS IN APOPTOTIC CELL DEATH OF NEUROEPITHELIAL CELLS
- PERSPECTIVES
- Figure
- Reference
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci 2009;3:31.
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci 2000;23:454-458.
- Kuan CY, Roth KA, Flavell RA, Rakic P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci 2000;23:291-297.
- Yeo W, Gautier J. Early neural cell death: dying to become neurons. Dev Biol 2004;274:233-244.
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol 2002;3:475-486.
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 2005;6:462-475.
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 1996;17:9-19.
- Xu Q, Wilkinson DG. Eph-related receptors and their ligands: mediators of contact dependent cell interactions. J Mol Med (Berl) 1997;75:576-586.
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci 2004;7:501-509.
- Yin Y, Yamashita Y, Noda H, Okafuji T, Go MJ, Tanaka H. EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci Res 2004;48:285-296.
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell 2005;121:127-139.
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature 2005;435:1244-1250.
- Holmberg J, Clarke DL, Frisen J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature 2000;408:203-206.
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet 2003;4:784-793.
- Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 1999;22:667-676.
- Park E, Kim Y, Noh H, Lee H, Yoo S, Park S. EphA/ephrin-A signaling is critically involved in region-specific apoptosis during early brain development. Cell Death Differ 2013;20:169-180.
- Kim Y, Park E, Noh H, Park S. Expression of EphA8-Fc in transgenic mouse embryos induces apoptosis of neural epithelial cells during brain development. Dev Neurobiol 2013;73:702-712.
- Lee H, Park E, Kim Y, Park S. EphrinA5-EphA7 complex induces apoptotic cell death via TNFR1. Mol Cells 2013;35:450-455.
- Bonanomi D, Chivatakarn O, Bai G, Abdesselem H, Lettieri K, Marquardt T, Pierchala BA, Pfaff SL. Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell 2012;148:568-582.
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O'Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron 2008;59:746-758.