Articles
Article Tools
Stats or Metrics
Article
Case Report
Exp Neurobiol 2016; 25(2): 93-101
Published online April 30, 2016
https://doi.org/10.5607/en.2016.25.2.93
© The Korean Society for Brain and Neural Sciences
Fractionated Stereotactic Gamma Knife Radiosurgery for Medial Temporal Lobe Epilepsy: A Case Report
Hye Ran Park1,3, Hyun-Tai Chung1, Sang Kun Lee2, Dong Gyu Kim1 and Sun Ha Paek1*
Department of 1Neurosurgery, 2Neurology, Seoul National University Hospital, Seoul 03080, 3Department of Neurosurgery, Soonchunhyang University Seoul Hospital, Seoul 04401, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-2072-3993, 82-2-2072-3957, FAX: 82-2-744-8459, 82-2-747-3799
e-mail: paeksh@snu.ac.kr
An 18-year-old left-handed male harbored intractable medial temporal lobe epilepsy (MTLE) underwent fractionated gamma knife surgery (GKS) instead of open surgery, considering the mental retardation and diffuse cerebral dysfunction. GKS treatment parameters were: target volume, 8.8 cm3; total marginal dose, 24 Gy in 3 fractionations at the 50% isodose line. The patient has been free from seizures since 9 months after GKS, with notable improvement in cognitive outcome. Fractionated GKS could be considered as a safe tool for seizure control and neuropsychological improvement in patients with MTLE.
Keywords: Radiosurgery, Stereotaxic Techniques, Epilepsy
INTRODUCTION
Although medical therapy is the mainstay for epilepsy, approximately 30% of patients are intractable [1]. The efficacy of open surgery for intractable medial temporal lobe epilepsy (MTLE) compared to prolonged medical therapy has been proven and it has become the treatment of choice for the treatment of intractable MTLE [2,3]. Long-term follow-up results of more than 5 years reported that the seizure-free rate after resection surgery was approximately 50–70% [4,5]. The mortality rate is less than 1%, and the rate of major complications ranges from 1 to 4% [6,7]. Since stereotactic radiosurgery revealed seizure control effect for cerebral arteriovenous malformations or tumors [8,9], gamma knife radiosurgery (GKS) has been proposed as an alternative option to open surgery for MTLE [10,11]. Non-invasiveness and safety are major advantages of GKS over open surgery [10]. On the other hand, delayed response and delayed radiation necrosis are suggested as a disadvantage of GKS [12]. For this reason, the clinical significance of GKS for MTLE has been the subject of controversy [13]. In this report, we report a case of an 18-year-old male patient with intractable MTLE treated with fractionated GKS, which reduced the adverse effects of single fractioned GKS and improved cognitive function with seizure free outcome.
CASE REPORT
The patient was an 18-year-old left handed male with a history of infantile febrile seizures that had spontaneously resolved at 8-years-of-age. He had no family history of seizures and was born by vaginal delivery without any perinatal problems. Complex partial seizures, sometimes with secondary generalization, started without specific cause at 14-years-of-age. The current seizure episodes were initiated by a rising sensation in the epigastrium followed by nausea and vomiting, loss of awareness, turning of his head to the left, and drooling. Episodes lasting within 1 minute occurred 2~4 times monthly. A variety of anti-epileptic drugs including topiramate, levetiracetam, rebamipide and oxcarbazepine were prescribed, but failed to control the seizures. He was referred to our institution for evaluation of temporal lobe surgery for the medical refractory seizures. He underwent preoperative evaluation including a neurological examination, scalp video-electroencephalogram (EEG) monitoring, high-resolution magnetic resonance imaging (MRI), 18F-deoxyglucose positron emission tomography (18FDG-PET), and a neuropsychological test.
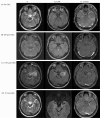
Ictal EEG demonstrated evolution from rhythmic delta wave to sharp wave in the left temporal area, suggesting left temporal onset epilepsy. A spike and intermittent semirhythmic slow activity in the left temporal area was shown in interictal EEG (Fig. 1). These findings were consistent with left temporal lobe epilepsy. Gadolinium-enhanced MRI showed left hippocampal sclerosis with slightly decreased volume and increased T2 signal (Fig. 2). The right hippocampus appeared normal, as did the remaining cerebral parenchyma. 18FDG-PET showed mild hypometabolism in the left temporal lobe compared to the right side. Neuropsychological test revealed moderate mental retardation (IQ, 50; VIQ, 55; PIQ, 59) and diffuse cerebral dysfunction in both frontal and temporal lobes (Table 1). He showed decreased attention ability with a borderline degree of impairment, and declined ability of storage and withdrawal memory. In language tests, spontaneous speech, auditory comprehension, reading, writing, and sequential commands were normal, but repetition and naming were impaired. Lateralization could not be clearly determined by the neurophysiologic test. Surgery was not considered due to the possibility of bilateral lesion and low intelligence. The epilepsy committee consisting of a neurologist, neurosurgeon, and others recommended GKS instead of open surgery. The patient underwent fractionated GKS after understanding that the seizures may or may not respond to treatment and that the antiepileptic drugs therapy would be continued for at least 2 years.
Fractionated GKS was performed under local anesthesia, using the Leksell Gamma Knife (model B Elekta Instrument AB) with Leksell Gamma Plan (Elekta, Stockholm, Sweden). Treatment planning was based on the combination of computed tomography (CT) and MRI to cover the amygdala, hippocampal head and body, most of the parahippocampal gyrus, and the entorhinal cortex (Fig. 3). The target region of 8.8 cm3 volume received marginal dose of total 24 Gy in 3 fractionations on 3 consecutive days with a dose of 8 Gy at the 50% isodose line (maximum dose 16.1 Gy). The patient tolerated the fixed frame for 3 days.
No acute or subacute periradiosurgical complications developed. He was maintained on anti-seizure medications including topiramate, levetiracetam, and oxcarbazepine. During the first month, he experienced 2 brief fits. However, the frequency of seizure episodes began to increase again at 1 month post-GKS. Although MRI obtained at post-GKS 6 months showed no significant interval change except slightly decreased volume of the lift hippocampus, the seizure frequency increased to reach 6 times per month at 9 months post-GKS. Afterward, further seizure activity or residual auras disappeared. At 11 months after GKS, the patient visited the hospital due to a severe headache without recurrence of seizure episodes. Follow-up T2-WI MRI revealed 2-cm sized round central low signals with surrounding high signals around the left hippocampus, which suggested radiation-induced changes (Fig. 1). The symptom was ameliorated by 10 days of steroid therapy.
An EEG performed 18 months after GKS revealed moderate amount of intermittent generalized theta slow activity without epileptiform discharges, suggesting diffuse cerebral dysfunction. He has continued to be seen by the neurologist and neurosurgeon and has remained free of complex-partial seizures of Engel class I. EEG obtained at 30 months after GKS showed no focal / generalized slow activity or epileptiform discharges, in accord with normal finding (Fig. 1). At 48 months after GKS, follow-up neuropsychological test and EEG were performed. In neuropsychological test, the patient showed improvement in general intelligence (IQ, 50→70; VIQ, 55→70; PIQ, 59→75), attention, memory, and motor function (Table 1). His general intelligence was improved from mild mental retardation to borderline. He did not show any significant change in language and frontal lobe function (Table 1). An EEG also was normal without epileptiform discharges. MRI performed 3 years after GKS showed disappearance of radiation necrosis. The patient has been free from seizures since 9 months after GKS, and the dosages of antiepileptic drugs have been tapered.
DISCUSSION
Since GKS for MTLE was first performed by Régis et al., it has been used as a less invasive treatment option for MTLE [14]. The authors reported that 65% of patients who underwent single fractionated GKS by 25 Gy to the 50% isodose obtained seizure-free at 2 years after GKS in a prospective multicenter study [15]. The Marseilles group also published 8-year follow-up results of 15 patients treated with 24 Gy to the 50% isodose line [16]. Nine of the patients obtained seizure-free, but seizure recurred in patients whose medication had been tapered or discontinued. Previous authors had suggested that radiosurgery induces biochemical changes resulting in a decrease of excitatory amino acids with gamma aminobyturic acid stability and inhibition of seizure production [14]. The results reported by Régis et al. as well as the multicenter, prospective pilot study in the Phase III Radiosurgery or Open Surgery for Epilepsy (ROSE) clinical trial suggested that the seizure outcome and complications of radiosurgery were similar comparable with those of open surgery [10,15,17].
On the other hand, Srikijvilaikul et al. failed to prove seizure control using a single fractionated GKS in 5 patients who underwent radiosurgery with 20 Gy to the 50% isodose [13]. Two patients died of a consequence of seizures, and the remaining 3 patients had no benefit until 1.5 years after radiosurgery. Other authors also reported no benefit from radiosurgery in terms of seizure control [12,18,19,20]. Low dose might be one of the causes of the discrepancy of seizure outcome in previous reports of unsatisfactory seizure outcome. In the study of Barbaro et al., the group randomized to 24 Gy had a higher proportion of seizure remission than those to randomized to 20 Gy for single fractionated GKS [10]. Radiosurgery can induce necrosis and consequent destruction of the epileptic focus and its pathways of spread. However, the dose of radiosurgery is a double-edged sword; single fractionated GKS with sufficiently high dose could obtain satisfactory seizure control effect, but it might lead to fatal adverse effects.
To resolve the dilemma double-edged sword fractionated GKS, which aims to integrate the advantages of fractionated conventional radiotherapy and single-session radiosurgery can be considered as an ideal alternative option for the control of medically refractory MTLE. Radiation delivered accurately permits larger doses to be delivered to larger areas without increasing the complication rate. Presently, the target volume of 8.8 cm3 contained the entire amygdala, hippocampal head and body, most of the parahippocampal gyrus, and the entorhinal cortex. In contrast, the target volume in previous studies included the amygdala sparing the upper and mesial part, the head and anterior half of the body of the hippocampus, and the anterior part of the parahippocampal gyrus [15,21]. Reducing dose per fraction could be an attractive alternative with minimizing adverse radiation effects and preserving the remaining function of medial temporal structures. As far as we know, this is the first case report of the use of fractionated GKS for MTLE, although Heikkinen et al. reported a patient with TLE who was successfully treated without any clinical or radiological complications by fractionated radiotherapy with a total dose equivalent to 10 Gy in 1992 [21].
Our patient obtained seizure control at 9 months after GKS. During the interval period between GKS and seizure remission, he experienced temporarily increased seizure frequency and underwent constant medical treatment. This delay in seizure control effects is one of the critical drawbacks of GKS for MTLE. In previous studies, the average duration until seizure control ranges from 10 to 12 months [10,16,17]. During this period, patients expose to unexpected mortalities associated with seizure. If a patient cannot tolerate the disabling seizures until seizure control, transition to open surgery should be considered.
The patient experienced mild degree of radiation induced changes in the follow up brain MRI at 11 months after GKS and symptoms relieved by short-term steroid therapy in contrast to the severe adverse reactions reported in the previous studies [10,12,13,14,18,19,20,22]. The radiological finding was accompanied by newly appeared headache, without recurrence of seizure episodes. Post-radiosurgical T2 hyperintensity lesion, contrast enhancement, and surrounding vasogenic edema have been reported to appear approximately 9 months to 1 year after GKS and gradually regressed within a few years [17,23]. At 24 months, the mass effects resolve to mild atrophy of the mesial temporal lobe. The radiation-induced MRI changes have been associated with the transient increase in seizure frequency, which did not happen with our case [12]. Newly developed headaches have been known to be noted in 70% of cases with post-radiosurgical MRI changes, and are well controlled by steroids [10].
The patient experienced unexpected favorable clinical outcomes in terms of cognitive function proved by neuropsychological test (Table 1). Little is known about the effect of GKS on cognitive functioning in TLE. Régis et al. have suggested that GKS may also produce less neuropsychological morbidity than standard surgery [14]. Srikijvilaikul et al. also reported no significant memory decline at a group level 6 months after radiosurgery, but they found significant declines in specific cognitive domains in 3 of 4 of their patients [13]. McDonald et al. reported on 3 patients treated on the language-dominant side during the period of development of the radiosurgical lesion. Significant impairment in at least one measure of verbal memory with sparing of IQ, visual memory, and language was evident [24]. A notable finding is that a worse neuropsychological outcome was found in patients irradiated with higher doses [12]. In the patients who obtained seizure freedom, quality of life is another important subject. Regarding this respect, our experience with this patient suggests the efficacy of the fractionated GKS to improve cognitive outcome in patients with MTLE.
This is the first case of fractionated GKS for MTLE. The patient achieved not only seizure control at 9 months after GKS, but also improvement of cognitive function. This case report suggests that fractionated GKS could be considered as a safe tool to achieve the better outcome in seizure control and neuropsychological improvement for patients with MTLE. Further long-term prospective studies with a larger numbers of patients are needed to determine the optimal doses and fraction schedules of fractionated GKS for MTLE.


K-WAIS-R, Korean-Wechsler Adult Intelligence Scale-Revised; WCST, Wisconsin Card Sorting Test; K-WAB, Korean version of Western Aphasia Battery; K-AVLT, Korean Auditory Verbal Learning Test; WMS-III, Wechsler Memory Scale-III; K-RCFT, Korean Rey Complex Figures Test; CPT, Continuous Performance Test; BDI, Beck Depression Inventory; HAM-D, Hamilton Rating Scale for Depression.
References
- Brodie MJ, Kwan P. Staged approach to epilepsy management. Neurology 2002;58:S2-S8.
- Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311-318.
- Spencer SS, Berg AT, Vickrey BG, Sperling MR, Bazil CW, Shinnar S, Langfitt JT, Walczak TS, Pacia SV, Ebrahimi N, Frobish D, Multicenter Study of Epilepsy Surgery. Initial outcomes in the multicenter study of epilepsy surgery. Neurology 2003;61:1680-1685.
- Elsharkawy AE, Alabbasi AH, Pannek H, Oppel F, Schulz R, Hoppe M, Hamad AP, Nayel M, Issa A, Ebner A. Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg 2009;110:1135-1146.
- Jutila L, Immonen A, Mervaala E, Partanen J, Partanen K, Puranen M, Kälviäinen R, Alafuzoff I, Hurskainen H, Vapalahti M, Ylinen A. Long term outcome of temporal lobe epilepsy surgery: analyses of 140 consecutive patients. J Neurol Neurosurg Psychiatry 2002;73:486-494.
- Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M, Poulin N, Arnold DL, Olivier A. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol 1996;40:446-450.
- Engel J, Van Ness PC, Rasmussen TB, Ojemann LM, Surgical treatment of the epilepsies. In: Engel J. . 2nd ed. New York, NY: Raven Press, 1993; 1993. p. 609-621.
- Eisenschenk S, Gilmore RL, Friedman WA, Henchey RA. The effect of LINAC stereotactic radiosurgery on epilepsy associated with arteriovenous malformations. Stereotact Funct Neurosurg 1998;71:51-61.
- Kida Y, Kobayashi T, Tanaka T, Mori Y, Hasegawa T, Kondoh T. Seizure control after radiosurgery on cerebral arteriovenous malformations. J Clin Neurosci 2000;7:6-9.
- Barbaro NM, Quigg M, Broshek DK, Ward MM, Lamborn KR, Laxer KD, Larson DA, Dillon W, Verhey L, Garcia P, Steiner L, Heck C, Kondziolka D, Beach R, Olivero W, Witt TC, Salanova V, Goodman R. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol 2009;65:167-175.
- Quigg M, Broshek DK, Barbaro NM, Ward MM, Laxer KD, Yan G, Lamborn K, Radiosurgery Epilepsy Study Group. Neuropsychological outcomes after Gamma Knife radiosurgery for mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia 2011;52:909-916.
- Vojtech Z, Vladyka V, Kalina M, Nešpor E, Seltenreichová K, Semnická J, Liscák R. The use of radiosurgery for the treatment of mesial temporal lobe epilepsy and long-term results. Epilepsia 2009;50:2061-2071.
- Srikijvilaikul T, Najm I, Foldvary-Schaefer N, Lineweaver T, Suh JH, Bingaman WE. Failure of gamma knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery 2004;54:1395-1402.
- Régis J, Kerkerian-Legoff L, Rey M, Vial M, Porcheron D, Nieoullon A, Peragut JC. First biochemical evidence of differential functional effects following Gamma Knife surgery. Stereotact Funct Neurosurg 1996;66:29-38.
- Régis J, Rey M, Bartolomei F, Vladyka V, Liscak R, Schröttner O, Pendl G. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia 2004;45:504-515.
- Bartolomei F, Hayashi M, Tamura M, Rey M, Fischer C, Chauvel P, Régis J. Long-term efficacy of gamma knife radiosurgery in mesial temporal lobe epilepsy. Neurology 2008;70:1658-1663.
- Régis J, Bartolomei F, Rey M, Hayashi M, Chauvel P, Peragut JC. Gamma knife surgery for mesial temporal lobe epilepsy. J Neurosurg 2000;93:141-146.
- Cmelak AJ, Abou-Khalil B, Konrad PE, Duggan D, Maciunas RJ. Low-dose stereotactic radiosurgery is inadequate for medically intractable mesial temporal lobe epilepsy: a case report. Seizure 2001;10:442-446.
- Kawai K, Suzuki I, Kurita H, Shin M, Arai N, Kirino T. Failure of low-dose radiosurgery to control temporal lobe epilepsy. J Neurosurg 2001;95:883-887.
- Prayson RA, Yoder BJ. Clinicopathologic findings in mesial temporal sclerosis treated with gamma knife radiotherapy. Ann Diagn Pathol 2007;11:22-26.
- Heikkinen ER, Heikkinen MI, Sotaniemi K. Stereotactic radiotherapy instead of conventional epilepsy surgery. A case report. Acta Neurochir(Wien) 1992;119:159-160.
- Barcia JA, Barcia-Salorio JL, López-Gómez L, Hernández G. Stereotactic radiosurgery may be effective in the treatment of idiopathic epilepsy: report on the methods and results in a series of eleven cases. Stereotact Funct Neurosurg 1994;63:271-279.
- Chang EF, Quigg M, Oh MC, Dillon WP, Ward MM, Laxer KD, Broshek DK, Barbaro NM, Epilepsy Radiosurgery Study Group. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology 2010;74:165-172.
- McDonald CR, Norman MA, Tecoma E, Alksne J, Iragui V. Neuropsychological change following gamma knife surgery in patients with left temporal lobe epilepsy: a review of three cases. Epilepsy Behav 2004;5:949-957.



