Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2016; 25(4): 185-190
Published online August 31, 2016
https://doi.org/10.5607/en.2016.25.4.185
© The Korean Society for Brain and Neural Sciences
Regional Cerebral Glucose Metabolism in Novelty Seeking and Antisocial Personality: A Positron Emission Tomography Study
So Hyeon Park1,2#, Hyun Soo Park1,2,3# and Sang Eun Kim1,2,3*
1Department of Transdisciplinary Studies, Graduate School of Convergence Science and Technology, Seoul National University, Seoul 16229, 2Department of Nuclear Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam 13620, 3Advanced Institutes of Convergence Technology, Suwon 16229, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-31-787-7671, FAX: 82-31-787-4018
e-mail: kse@snu.ac.kr
#So Hyeon Park and Hyun Soo Park contributed equally to the present study in the experimental design, manuscript writing, and discussion.
Novelty seeking (NS) and antisocial personality (ASP) are commonly exhibited by those who suffer from addictions, such as substance abuse. NS has been suggested to be a fundamental aspect of ASP. To investigate the neurobiological substrate of NS and ASP, we tested the relationship between regional cerebral glucose metabolism and the level of NS, determining the differences between individuals with and without ASP. Seventy-two healthy adults (43 males, mean age±SD=38.8±16.6 years, range=20~70 years; 29 females, 44.2±20.1 years, range=19~72 years) underwent resting-state brain positron emission tomography (PET) 40 minutes after 18F-fluorodeoxyglucose (FDG) injection. Within 10 days of the FDG PET study, participants completed Cloninger's 240-item Temperament and Character Inventory (TCI) to determine NS scores. Participants with and without ASP were grouped according to their TCI profiles. Statistical parametric mapping analysis was performed using the FDG PET and TCI profile data. NS scores positively correlated with metabolism in the left anterior cingulate gyrus and the insula on both sides of the brain and negatively correlated with metabolism in the right pallidum and putamen. Participants with ASP showed differences in cerebral glucose metabolism across various cortical and subcortical regions, mainly in the frontal and prefrontal areas. These data demonstrate altered regional cerebral glucose metabolism in individuals with NS and ASP and inform our understanding of the neurobiological substrates of problematic behaviors and personality disorders.
Keywords: Neural Substrate of Personality, Novelty Seeking, Antisocial Personality, FDG PET, Statistical Parametric Mapping (SPM)
INTRODUCTION
Previous studies using Cloninger's temperament and character model [1] have provided important information for investigating individual differences in cognitive behaviors and basic stimulus-response characteristics [1,2]. In this model, personality is divided into the categories of temperament and character. Temperament primarily consists of four dimensions: novelty seeking (NS), harm avoidance, reward dependence, and persistence. Character reflects acquired behavioral, emotional, and thought patterns and has three dimensions: self-directedness, cooperativeness, and self-transcendence. Cloninger's Temperament and Character Inventory (TCI) has been used to assess personality profiles and consists of seven dimensions based on the socio-biological personality model. As a biological basis of personality, the TCI profile is known to indicate an individual's propensity for mental abnormalities or problematic behaviors [3,4,5,6,7]. The TCI profile also has neurological implications for healthy individuals, as well as those with personality disorders associated with abnormal neurotransmitter activity [5,8]. In particular, several lines of evidence suggest that dopaminergic neurotransmission is involved in NS [5,9]. For example, human genetic studies have reported that dopamine D4 receptor [10,11] and dopamine D2 receptor polymorphisms were associated with NS [12]. NS has been found in substance abusers, and it is associated with dopamine D2 receptors on both the genetic and endocrine levels [3,4,5,13].
A previous study reported that high NS predisposes individuals to adult antisocial personality disorder [1,14,15] that is closely related to a hypersensitive brain reward system modulated by the dopamine neurotransmitter systems [16]. NS involves behavioral activation and describes a genetic disposition towards being excitable, impulsive, exploratory, and quick-tempered. Antisocial personality disorder is characterized by a pervasive pattern of disregard for, or violation of, the rights of others. Increasing evidence indicates that deficits in prefrontal functioning are characteristic of violent, antisocial persons as indicated by both positron emission tomography (PET) [17,18] and single-photon emission computed tomography [19].
In the present study, we investigated the relationship between regional cerebral glucose metabolism and NS scores, and the differences in level of regional cerebral glucose metabolism (cerebral activation or deactivation) of individuals with ASP compared to those without ASP using FDG PET and TCI profile data acquired from healthy individuals.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of the Seoul National University College of Medicine and the Seoul National University Bundang Hospital. All participants were informed about the study procedures in detail and subsequently provided written informed consent for their participation.
Participants were recruited by community bulletin boards and internet advertisements. Seventy-two right-handed healthy volunteers (43 males, mean age±SD=38.8±16.6 years, range=20~70 years; 29 females, 44.2±20.1 years, range=19~72 years) participated. Researchers obtained a self-reported clinical history for each participant in order to exclude individuals with neurological abnormalities that might affect brain metabolism. All participants answered that they did not have any neurological or psychiatric history and that they were drug-free.
We used a Korean version of the TCI with 240 items [20] to assess participants' TCI profile. Within 10 days after the FDG PET study, participants completed a TCI of seven dimensions. Based on individual TCI profiles, participants were allocated into one of eight personality categories (antisocial, histrionic, passive-aggressive, explosive, obsessional, schizoid, cyclothymic, and passive-dependent) using quartile statistics [12,13]. First and second quartile scores were defined as low scores, and third and fourth quartile scores were defined as high scores for each personality dimension [2]. Finally, of the 72 participants, 18% (
PET data were acquired using an Allegro PET scanner operating in the three-dimensional (3D) mode. Participants fasted for at least 6 hours before scanning. Prior to imaging, participants were administered an intravenous injection of 4.8 MBq/kg FDG in a dimly lit, quiet waiting room and were instructed to remain lying comfortably during a 40-minute FDG equilibration period. Participants were then led to an adjacent imaging suite and positioned within the PET scanner so that the head was aligned relative to the canthomeatal line. Ten-minute emission scans and attenuation maps using a Cs-137 transmission source were obtained. Attenuation-corrected images were reconstructed using PET data and a 3D row-action maximum-likelihood algorithm with a 3D image filter of 128×128×90 matrices and a pixel size of 2×2×2 mm.
Spatial preprocessing and statistical analyses were performed using SPM2 (Institute of Neurology, University College of London, UK) implemented in MATLAB (Mathworks Inc., Natick, MA, USA). Every image was spatially normalized to the standard MNI template (Montreal Neurological Institute, McGill University, CA) of SPM2 in order to eliminate within-subject anatomical variability [21]. Spatially normalized images were smoothed by convolution with an isotropic Gaussian kernel with 12 mm full width at half maximum to increase the signal-to-noise ratio and accommodate variations in subtle anatomical structures. Glucose metabolism of each voxel was also normalized by using proportional scaling with the mean glucose metabolism of global gray matter set at 100.
To identify regions showing relationships between resting-state patterns of regional cerebral glucose metabolism and the NS scores, SPM correlation analyses were performed for each voxel with a general linear approach. NS scores were incorporated as covariates of interest, whereas age was set as nuisance variable to regress out its effects. The height threshold (
Neurobiological differences in ASP were investigated using the mean image of the ASP group and the mean image of all other individuals. The height threshold (
Results and Discussion
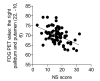
The cerebral metabolic correlates of NS are provided in Table 1. Positive correlations between cerebral glucose metabolism and NS scores were found in the left anterior and middle cingulate gyrus (peak activation at
Participants with ASP (13 of the 72 participants, 18%) showed significantly higher NS scores (mean±SD=21.3±3.3) than those without ASP (mean±SD=15.6±5.6;
In the present study, we investigated the neurobiological substrates of NS and ASP as fundamental aspects of problematic behavior. Voxel-based correlation and subtraction analysis with TCI scores and FDG PET data revealed that personality variants might be predicted by resting-state patterns of regional cerebral glucose metabolism. These data may benefit the identification of patients who are at risk for the development of psychological or psychiatric disorders.

| Region | Clusters ( | Coordinates | Z scores | |||
|---|---|---|---|---|---|---|
| Positive correlations with NS score | ||||||
| L | Anterior cingulate gyrus | 1996 | −4 | 32 | 14 | 4.41 |
| R | Middle cingulate gyrus | 8 | 18 | 30 | 4.22 | |
| R | Insula | 835 | 38 | 20 | −10 | 3.49 |
| L | Insula | 581 | −44 | −10 | −4 | 4.10 |
| Negative correlations with NS score | ||||||
| R | Pallidum | 195 | 22 | −10 | 6 | 3.63 |
| R | Pallidum | 28 | 0 | 14 | 3.31 | |
L, left; NS, novelty seeking; R, right. p<0.001, uncorrected;
| Region | Clusters ( | Coordinates | Z scores | |||
|---|---|---|---|---|---|---|
| Activated regions in ASP | ||||||
| L | Inferior frontal gyrus | 156 | −54 | 14 | 4 | 3.02 |
| L | Middle cingulate gyrus | 161 | −2 | 16 | 32 | 2.99 |
| L | Medial superior frontal gyrus | 180 | 0 | 46 | 18 | 2.80 |
| Deactivated regions in ASP | ||||||
| L | Supramarginal gyrus | 2211 | −48 | −24 | 26 | 4.53 |
| R | Superior orbitofrontal gyrus | 814 | 26 | 42 | −2 | 4.00 |
| L | Middle temporal gyrus | 1090 | −48 | −44 | 2 | 3.78 |
| R | Hippocampus | 744 | 40 | −22 | −16 | 3.60 |
| L | Postcentral gyrus | 1115 | −22 | −36 | 58 | 3.50 |
| L | Middle cingulate gyrus | 245 | −18 | −52 | 38 | 3.43 |
| R | Middle cingulate gyrus | 159 | 14 | 18 | 38 | 3.21 |
| L | Middle cingulate gyrus | 150 | −36 | −68 | 10 | 3.10 |
ASP, antisocial personality; L, left; R, right. p<0.005 uncorrected,
References
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry 1987;44:573-588.
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry 1993;50:975-990.
- Compton PA, Anglin MD, Khalsa-Denison ME, Paredes A. The D2 dopamine receptor gene, addiction, and personality: clinical correlates in cocaine abusers. Biol Psychiatry 1996;39:302-304.
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, Kurian V, Kimes AS, Breeden A, Grant S. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res Cogn Brain Res 2005;23:119-136.
- Laine TP, Ahonen A, Räsänen P, Tiihonen J. Dopamine transporter density and novelty seeking among alcoholics. J Addict Dis 2001;20:91-96.
- Cloninger CR, Svrakic DM, Przybeck TR. Can personality assessment predict future depression? A twelve-month follow-up of 631 subjects. J Affect Disord 2006;92:35-44.
- Hansenne M, Reggers J, Pinto E, Kjiri K, Ajamier A, Ansseau M. Temperament and character inventory (TCI) and depression. J Psychiatr Res 1999;33:31-36.
- Peirson AR, Heuchert JW, Thomala L, Berk M, Plein H, Cloninger CR. Relationship between serotonin and the temperament and character inventory. Psychiatry Res 1999;89:29-37.
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci 2008;28:14372-14378.
- Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nat Genet 1996;12:81-84.
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat Genet 1996;12:78-80.
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 1991;48:648-654.
- Wiesbeck GA, Mauerer C, Thome J, Jakob F, Boening J. Neuroendocrine support for a relationship between "novelty seeking" and dopaminergic function in alcohol-dependent men. Psychoneuroendocrinology 1995;20:755-761.
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. Br J Psychiatry 2005;186:190-196.
- Hesselbrock MN, Hesselbrock VM. Relationship of family history, antisocial personality disorder and personality traits in young men at risk for alcoholism. J Stud Alcohol 1992;53:619-625.
- Ponce G, Jimenez-Arriero MA, Rubio G, Hoenicka J, Ampuero I, Ramos JA, Palomo T. The A1 allele of the DRD2 gene (TaqI A polymorphisms) is associated with antisocial personality in a sample of alcohol-dependent patients. Eur Psychiatry 2003;18:356-360.
- Goyer PF, Andreason PJ, Semple WE, Clayton AH, King AC, Compton-Toth BA, Schulz SC, Cohen RM. Positron-emission tomography and personality disorders. Neuropsychopharmacology 1994;10:21-28.
- Raine A, Meloy JR, Bihrle S, Stoddard J, LaCasse L, Buchsbaum MS. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behav Sci Law 1998;16:319-332.
- Kuruoğlu AC, Arikan Z, Vural G, Karataş M, Araç M, Işik E. Single photon emission computerised tomography in chronic alcoholism. Antisocial personality disorder may be associated with decreased frontal perfusion. Br J Psychiatry 1996;169:348-354.
- Sung SM, Kim JH, Yang E, Abrams KY, Lyoo IK. Reliability and validity of the Korean version of the temperament and character inventory. Compr Psychiatry 2002;43:235-243.
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York, NY: Thieme, 1988.
- Sugiura M, Kawashima R, Nakagawa M, Okada K, Sato T, Goto R, Sato K, Ono S, Schormann T, Zilles K, Fukuda H. Correlation between human personality and neural activity in cerebral cortex. Neuroimage 2000;11:541-546.
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp 2009;30:1188-1195.
- Goldstein RZ, Alia-Klein N, Volkow NS, Larry RS. In: Squire LR. Encyclopedia of neuroscience. Oxford: Academic Press, 2009; 2009. p. 699-711.
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry 2006;60:383-387.
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 2007;164:318-327.
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry 2007;62:642-651.
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci 2007;27:14035-14040.
- Sarinopoulos I, Dixon GE, Short SJ, Davidson RJ, Nitschke JB. Brain mechanisms of expectation associated with insula and amygdala response to aversive taste: implications for placebo. Brain Behav Immun 2006;20:120-132.
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 2009;10:59-70.
- Weiger WA, Bear DM. An approach to the neurology of aggression. J Psychiatr Res 1988;22:85-98.
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport 2001;12:2595-2599.
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage 2003;19:1085-1094.
- Soloff PH, Meltzer CC, Becker C, Greer PJ, Kelly TM, Constantine D. Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatry Res 2003;123:153-163.



