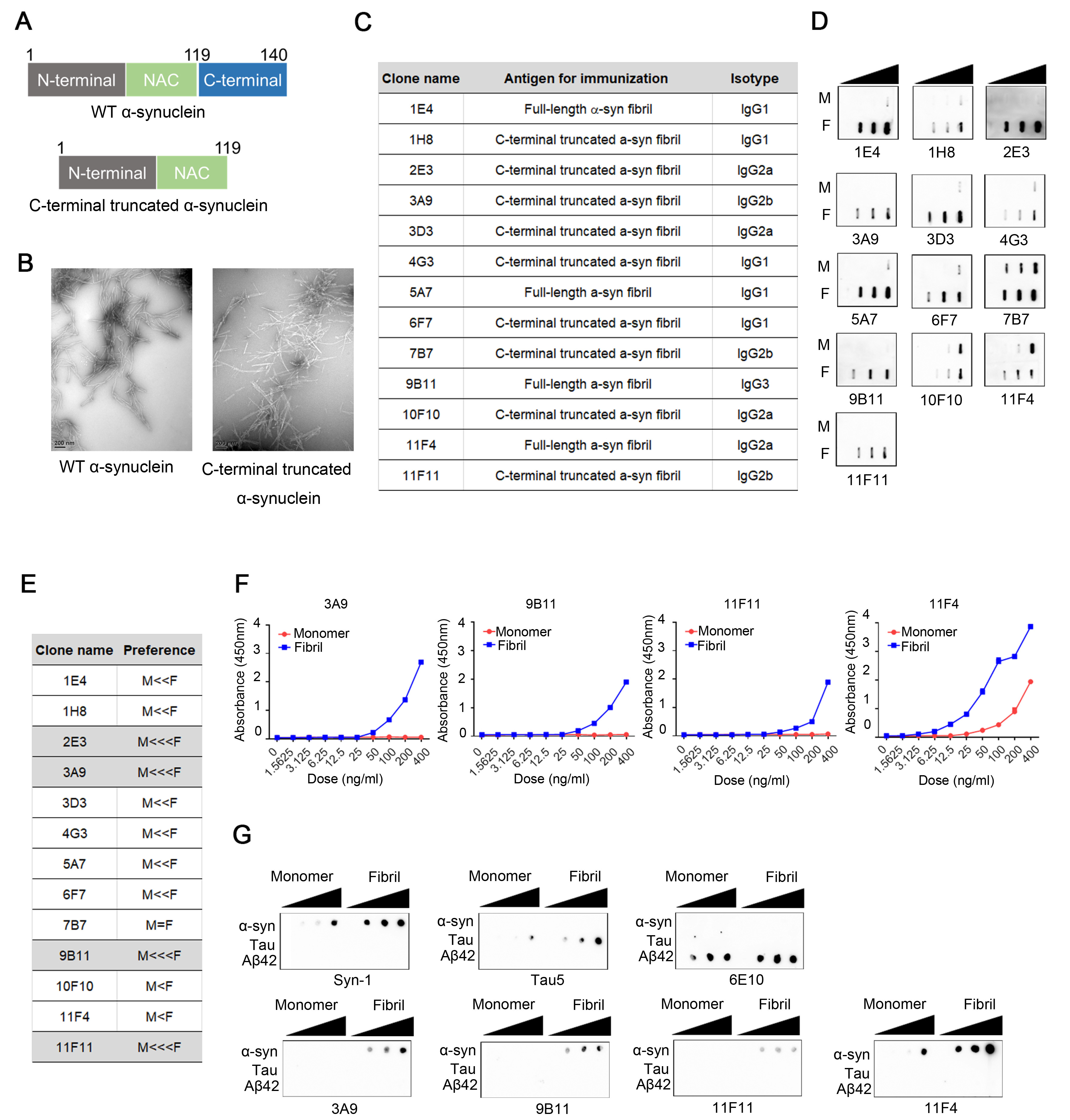
Fig. 1. Characterization of α-synuclein antibodies. (A) Antigens for immunization for production of antibodies. (B) Ultrastructural image of the proteins in (A). (C) Information on immunogens and isotypes of resulting antibody clones. Conformation-specific antibodies were generated by immunization of C57BL6 mice with either full-length α-synuclein or C-terminally truncated α-synuclein. (D) Specificity for monomer (M) or fibril (F) in dot blots. (E) Preference for aggregated forms of α-synuclein based on (D). The relative affinity towards M and F forms is indicated by the number of ‘<’ symbols. “M <<< F” antibodies were selected for further assay. (F) Affinity of antibodies for monomer and aggregated forms of α-synuclein, determined by sandwich ELISA. (G) Cross-reactivity with amyloid β42 and tau proteins. Two-fold serial dilutions (from 50 ng) of both monomeric and aggregated proteins were spotted onto a nitrocellulose membrane. Syn-1, Tau5 and 6E10 antibodies were used as positive controls for α-synuclein, tau, and amyloid β42 respectively.
© Exp Neurobiol


