Articles
Article Tools
Stats or Metrics
Article
Original Research
Exp Neurobiol 2010; 19(1): 54-61
Published online June 30, 2010
https://doi.org/10.5607/en.2010.19.1.54
© The Korean Society for Brain and Neural Sciences
Finger Motion Decoding Using EMG Signals Corresponding Various Arm Postures
Kyung‐Jin You, Ki‐Won Rhee and Hyun‐Chool Shin*
Department of Electronic Engineering, College of IT, Soongsil University, Seoul 156‐743, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-828-7165, FAX: 82-2-821-7653
e-mail: shinhc@ssu.ac.kr
Abstract
We provide a novel method to infer finger flexing motions using a four-channel surface electromyogram (EMG). Surface EMG signals can be recorded from the human body non-invasively and easily. Surface EMG signals in this study were obtained from four channel electrodes placed around the forearm. The motions consist of the flexion of five single fingers (thumb, index finger, middle finger, ring finger, and little finger) and three multi.finger motions. The maximum likelihood estimation was used to infer the finger motions. Experimental results have shown that this method can successfully infer the finger flexing motions. The average accuracy was as high as 97.75%. In addition, we examined the influence of inference accuracies with the various arm postures.
Keywords: surface EMG, finger motions, neural signal processing, HCI
INTRODUCTION
Compared to the invasive type electromyogram (EMG) for measuring each motor unit action potential (MUAP) on muscle fiber in a small area, surface EMG signals can be recorded from the human body non-invasively and easily over wide skin areas. To extract the motion information, surface EMG signal processing has been studied, especially focusing on controlling electronic devices such as a human supporting prosthetic arms and smart interface (Sornmo and Laguna, 2005). Since motor commands are transmitted through internal nerve tissues or muscles, inferring such commands only from the surface EMG signals is not an easy task.
Thus many attempts to extract motor information from surface EMG have been made. A study of Englehart et al. (2001) used wavelet analysis and PCA analysis to classify two arm motions and two wrist motions. Also, Englehart and Hudgins (2003) used the absolute mean value, the zero crossing rate, and the wavelength to classify four arm and wrist motions. Momen et al. (2007) used RMS and FCMs to classify arm and wrist motions. Hudgins et al. (1993) obtained an ensemble average of EMG signals, and used a neural networks (NN) to classify four arm motions.
While the surface EMG-based motion inference has been focused on upper limb or hand motions, studies on inferring dexterous finger motions have not been done so much. Nishikawa et al. (1999) used the Gabor transform and absolute mean value to extract the features and classify four wrist motions and six finger motions in real-time learning based on NN. Nagata et al. (2007) used absolute sum analysis, canonical component analysis, and minimum Euclidean distance to classify four wrist motions and five finger motions. Uchida et al. (1992) used FFT analysis and NN to classify four finger motions. Chen et al. (2007) used mean absolute values (MAV), the ratio of the MAVs, autoregressive (AR) model, and linear Bayesian classifier to classify 5~16 finger motions. However, NN classifiers (Hudgins et al., 1993; Chen et al., 2007; Nagata et al., 2007) have strong dependency on initial parameter conditions. Also, linear classifiers (Uchida et al., 1992; Englehart et al., 2001; Englehart and Hudgins, 2003; Momen et al., 2007; Nagata et al., 2007) are simple but show low accuracy compared with nonlinear classifiers.
In this paper, we propose a novel method to infer the finger motions using surface EMG signals. We recorded the EMG signals during finger flexing motions. To infer the finger motions, we used the maximum likelihood method. The likelihood was obtained by building probabilistic models of EMG activities. The information entropy of the EMG magnitude was calculated to quantify the EMG activities. Experimentally we demonstrated that the proposed method could infer the finger flexing motions including multi-fingers as high as 97.75%.
In addition, we examined the influence of inference accuracies with various arm postures.
A signal acquisition device (Poly-G-A, Laxtha Inc., Korea), bi-polar Ag-AgCl electrodes (Dual electrode #272, Noraxon U.S.A. Inc.), and snap electrodes (SECS-4, Laxtha Inc., Korea) were used. The EMG signal was observed at the sampling frequency of 512 Hz and was filtered to reduce power line noise.
Two able-bodied subjects without any upper limb deficiencies participated in performing motions for this study and was trained before signal recording. The subjects were instructed to maintain their own flexing speed and strength.
The EMG signal was acquired with four channels on the left forearm (around the wrist) for 3.0 seconds. Fig. 1 shows electrode orientation for EMG recording. During the first 1.5 seconds, the subject did not perform any motion - termed 'relaxation' - and waited for an acoustic alarm. After 1.5 seconds, the subject heard the acoustic alarm and then flexed a finger immediately. After 3.0 seconds, the EMG signal recording procedure ended. In the experiment, the subject performed eight finger flexing motions including three multi-finger motions. Fig. 2 shows the detail of the finger motions. The subject took a long and sufficient rest between recording procedures to avoid muscle fatigue or cramp.
PROPOSED METHOD
Here we consider four EMG channels and eight finger flexing motions. Let
Since the EMG signal has both positive and negative levels, we take an absolute of

For example, Fig. 3 shows the surface EMG signal of the 2nd channel during the
The amplitude of the EMG signal is a fundamental quantity which increases monotonically with the force developed in the muscle (Sornmo and Laguna, 2005). Moreover, the EMG signal represents a stochastic signal (Sornmo and Laguna, 2005).
The entropy (information entropy, H) was used to represent the characteristics of each EMG signal activity during finger flexing motions. The entropy is a measure that can reveal how much dynamical nature and the information (Shannon and Weaver, 1963) which the signal contains. Some EEG analysis methods based on entropy have been developed. (Capurro et al., 1999; Martin et al., 2000; Tong et al., 2002; Shin et al. 2006; Sabeti et al., 2009) Although the amplitude range or the spectral bandwidth of EMG and EEG signal are remarkably different, but they have a non- stationary stochastic property in common.
Conventionally the entropy is calculated as follows:
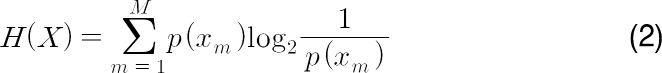
where
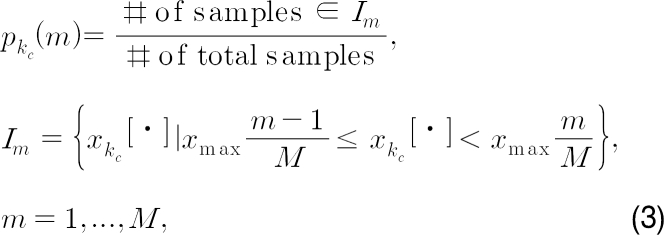
where
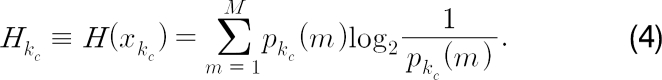
After accumulating the entropy in eq. (4), we build the probability density function of the entropy based on the Gaussian model:
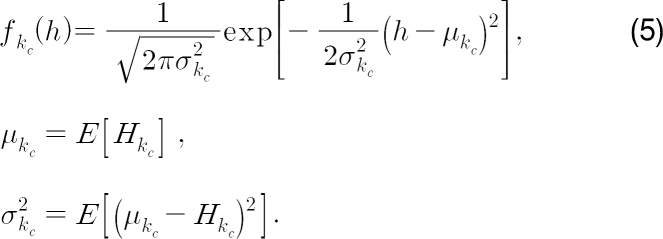
Here

Then, we find

where
EXPERIMENTAL RESULTS
A cross-validation is used to estimate the inference accuracies. The number of training data is 25 and that of the test data is 25. All test data are excluded from the training data. The results are averaged over 500 combinations.
The accuracy for six motions (
Fig. 6 shows Likelihood for various candidates of finger motions (each value is normalized so that the maximum was equal to one.
(a)
(c)
(e)
(g)
The average inference accuracy for four motions (flexion of thumb, flexion of index finger, flexion of middle finger, flexion of all fingers) in a study (Uchida et al., 1992) was 86% and the accuracy for six motions (extension of thumb, extension of index finger, extension of middle finger, extension of ring finger, extension of little finger, and 'hook' gesture) was 96.83% (Chen et al., 2007).
The inference results for eight motions (
We set nine arm postures to find out how much accuracies hold down when human changes arm posture. Fig. 8 shows basic postures.
Matrices on the diagonal site in Fig. 9 show inference accuracies for five single-finger flexion motions of each posture; the number of training data was 25 and that of test data was equal. Each experiment was done 50 times. All test data were excluded from the training data. The average inference accuracies are 74.72%, 83.78%, 76.22%, 81.15%, 83.60%, 62.62%, 63.07%, 76.83%, and 77.55%, respectively.
We tested again with the data of another postures as a training dataset. Other matrices in Fig. 9 show the results. The average inference accuracies are shown in Table 4. The shaded cells indicate the maximum average accuracy in the same row and these match the diagonal cells.
Accordingly, the data of an posture is not appropriate to the reference of other postures. This fact indicates that a robust finger motion decoding method cannot be implemented without a scheme detects changes of arm's posture.
DISCUSSION
We have proposed a new method to infer finger flexing motions based on the entropy and the maximum likelihood estimation. The average recognition accuracy for six finger motions was 100.00% and the accuracy for eight finger motions was 97.75%. Also we have shown the quantitative interpretation of the need of avoidance of use of reference dataset cross arm posture because that influences the correlations between the EMG signals and the performed finger flexing motions. This study may trigger a new type of human-computer interface with user's intuitive hand motions which could control electronic devices.
Table 1. Inference accuracy (%) for six motions (subject A,
References
- Capurro A, Diambra L, Lorenzo D, Macadar O, Martin MT, Mostaccio C, Plastino A, Perez J, Rofmane E, Torres ME, Velluti J. Human brain dynamics: the analysis of EEG signals with Tsallis information measure. Physica A 1999;265:235-254.
- Chen X, Zhang X, Zhao Z, Yang J, and Wang K 2007. Multiple Hand Gesture Recognition based on Surface EMG Signal. The 1st International Conference on Bioinformatics and Biomedical Engineering, Wuhan, China, pp.506-509.
- Englehart K, Hudgins B. A robust, real-time control scheme for multifunction myoelectric control, IEEE Trans. On Biomed Eng 2003;50:848-854.
- Englehart K, Hudgins B, Parker PA. A Wavelet-Based Continuous Classification for Multifucntion Myoelectric Control, IEEE Trans. On Biomed Eng 2001;48:302-311.
- Hudgins B, Parker P, Scott R. A new strategy for multifunction myoelectric control, IEEE Trans. On Biomed Eng 1993;40:82-94.
- Martin MT, Plastinob AR, Plastino A. Tsallis-like information measures and the analysis of complex signals. Physica A 2000;275:262-271.
- Momen K, Krishnan S, Chau T. Real-Time Classification of Forearm Electromyographic signals Corresponding to User-Selected Intentional Movements for Multifunction Prosthesis Control, IEEE Trans. On Neural Systems and Rehabilitation Engineering 2007;15:535-542.
- Nagata K, Ando K, Magatani K, and Yamada M 2007. Development of the hand motion recognition system based on surface EMG using suitable measurement channels for pattern recognition. Conf. Proc. of the IEEE Trans. On Eng Med Biol Soc, pp.5214-5217.
- Nishikawa D, Yu W, Yokoi H, and Kakazu Y 1999. EMG Prosthetic Hand Controller Discriminating Ten Motions using Real-time Learning Method. IEEE/RSJ Intl. Conf. On Intelligent Robots and Systems, Kyongju, South Korea, pp.1592-1597.
- Sabeti M, Katebi S, Boostani R. Entropy and complexity measures for EEg signal classification of schizophrenic and control participants. Artif Intell Med 2009;47:263-274.
- Shannon CE, Weaver W. The mathematical theory of communication. Urbana, Illinois: University of Illinois Press, 1963.
- Shin HC, Tong S, Yamashita S, Jia X, Geocadin RG, Thakor NV. Quantitative EEG and effect of hypothermia on brain recovery after cardica arrest, IEEE Trans. On Biomed Eng 2006;53:1016-1023.
- Sornmo L, Laguna P. Bioelectrical signal processing in cardiac and neurological applications. London: Elsevier Academic Press, 2005.
- Tong S, Bezerianos A, Paul J, Zhu Y, Thakor N. Nonextensive entropy measure of EEG following brain injury from cardiac arrest. Physica A 2002;305:619-628.
- Uchida N, Hiraiwa A, Sonehara N, and Shimohara K 1992. EMG Pattern Recognition by Neural Networks for Multi Fingers Control. Proceedings of the Annual International Conference of the IEEE. On Engineering in Medicine and Biology Society, pp.1016-1018.












