Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2013; 22(3): 214-223
Published online September 30, 2013
https://doi.org/10.5607/en.2013.22.3.214
© The Korean Society for Brain and Neural Sciences
Beneficial Effect of Vitamin E in Rotenone Induced Model of PD: Behavioural, Neurochemical and Biochemical Study
Neha Sharma and Bimla Nehru*
Department of Biophysics, Panjab University, Chandigarh 160014, India
Correspondence to: *To whom correspondence should be addressed.
TEL: 91-172-2534128, FAX: 91-172-2534128
e-mail: nehrubimla14@gmail.com, bnehru@pu.ac.in
Abstract
Parkinson's disease (PD) a neurodegenerative disorder for which no preventive or long-term effective treatment strategies are available. Epidemiologic studies have failed to identify specific environmental, dietary or lifestyle risk factors for PD. However, oxidative stress in the SN is the most broadly accepted hypothesis for the etiopathology of PD. The Symptoms do not appear until there is a decline of striatal dopamine levels by 80% making it difficult to have early therapeutic interventions. Thus, the present experiment was designed to track down the sequential changes starting from the initiation of motor dysfunction and associated biochemical abnormality in rotenone based PD model. The study also evaluated the neuroprotective efficacy of vitamin E. Rats were treated with rotenone 2 mg/kg b.wt (s.c.) for 35 days. The level of dopamine decreased by 70~80% which was in turn reflected by marked deterioration in motor function such as (Total locomotor activity and catalepsy). Along with these the level of GSH and SOD declined significantly which was associated with elevated lipid peroxidation levels as much as by 60%.Vitamin E co-administration at a dose of 100 I.U/kg b.wt (i.m.) ameliorated rotenone induced changes in motor functions i.e Total locomotor activity and Catalepsy at the end of 5th week. Further, vitamin E supplementation significantly decreased lipid peroxidation and improved associated biochemical parameters i.e SOD and GSH level. Most interestingly the changes appeared as early as 3rd week suggesting that supplementation of vitamin E right at the beginning should be neuroprotective in PD.
Keywords: Parkinson's disease, substantia nigra, oxidative stress, pars compacta, rotenone, catalepsy
INTRODUCTION
Parkinson's disease (PD) is one of the leading causes of neurologic disability in elderly [1]. Its pathological hallmark is the specific and progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) [2] which results in extrapiramidal motor dysfunction accompanied by progressive impairment of autonomy, mood, and cognitive functions [3-5].
With the misdiagnosis rate of about 24% [6] and appearance of symptoms at (>80%) depletion in striatal dopamine levels [7], it becomes difficult to diagnose PD at an early stage in humans. Whereas, animal models allow us to study the pathology of PD while tracking physical and behavioural changes during the entire disease course. Amongst the available animal models (eg. MPTP, 6-OHDA, Reserpine, Paraquat etc) the rotenone model depicts the classical features of PD [8] moreover due to its use as an organic pesticide has stimulated significant interest in this model of PD. Rotenone, commonly used is a potent specific inhibitor of mitochondrial complex I [9]. Due to its lipophylic nature it can easily cross the blood brain barrier. Rats administered with subacute doses of rotenone develop biochemical, anatomical and behavioural symptoms similar to that observed in PD [8, 9]. It has been reported that chronic systemic or subcutaneous pesticide exposure reproduces characteristic features of Parkinson's disease [9] producing highly selective dopaminergic degeneration and alpha-synuclein aggregation [10].
The major problem concerning a better therapeutic approach to the treatment and prevention of PD is the enigma of its underlying cause. The culprit is less likely to be a single cause than a combination of genetic and biological factors, which are triggered by some environmental assaults. Generation of Oxidative stress (OS) as a result of dopamine metabolism and its self oxidation, mitochondrial dysfunction as a result of complex I inhibition [11, 12] activation of microglial NADPH oxidase produced superoxide anion and cytokines (TNF-α and IL-1β) [13] consequently leads to the activation of apoptotic cascades in SN neurons leading to Dopaminergic neurodegeneration. Thus, suggesting that antioxidant supplementation could be helpful in developing therapeutic strategies for PD.
Antioxidants (like vitamins, polyphenols, lipoic acid etc) as Nutrient supplementation have already been used in clinical studies and are being widely used by people with Parkinson's disease in order to partially treat or slow down the deterioration. Vitamin E is such naturally occurring, lipid soluble, chain breaking antioxidant in biological membranes [14]. Since vitamin E is an effective free radical scavenger in the brain its neuroprotective function is the issue of new therapeutic approaches in neurodegenerative diseases. Biochemical evidences showed that alpha-tocopherol prevented the toxin-induced destruction of striatal DA terminals [15, 16]. Also there is a dose dependent inverse association between vitamin E dietary consumption and PD incidence [17]. It was showed that vitamin E prevented neuronal damage from reactive NO species and was found to play an important role in neurodegenerative diseases related to oxidative stress like Alzheimer's disease, Parkinson's disease and Huntington's disease [18-20].
From the above mentioned references, vitamin E as a nutritional supplement, can serve to be a potent neuroprotector. For a better understanding of the disease progression process present study was undertaken to evaluate the sequential changes during the course of PD development following rotenone exposure and the mechanistic evaluation of the neuroprotective efficacy of vitamin E during the progressive deterioration of Dopaminergic neurons.
EXPERIMENTAL METHODOLOGY
Chemicals
All the chemicals were of analytical grade. Rotenone was purchased from Sigma (St. Louis, USA), Vitamin E from Merck and other chemicals from Sisco Research Laboratories Pvt Ltd (Mumbai India) and Hi-Media Chemicals.
Animals
Healthy Male rats of the Sprague Dawley strain of 5~7 weeks age, weighing 200~250 grams were procured from the central animal house of the university and were housed in polypropylene cages under hygienic conditions and were provided free access to standard animal feed (Ashirwad Industries, Ropar, India) and water ad libitum throughout the treatment period. All procedures were performed in accordance with ethical guidelines on the care and use of laboratory animals which were approved by Institutional Animal Ethics Committee (IAEC).
Experimental design
Animals were randomly divided into 4 groups with 12~14 animals in each group. Behavioural parameters were recorded weekly from the start of experiment till the end of 5th week. After 3 weeks of treatment 6 animals were randomly selected from each group and were sacrificed for estimation of biochemical parameters. Remaining animals were sacrificed after 2 weeks of further treatment to record the alterations.
Grouping of animals was done as follows:
Group I: Control
Animals were administered with vehicle (sunflower oil) for a period of 5 weeks (35 days).
Group II: Rotenone treated
Animals in this group were administrated with rotenone (2 mg/kg body weight s.c.) dose dissolved in sunflower oil daily for a period of 5 weeks (35 days).
Group III: Vitamin E treated
Animals in this group were administered vitamin E (100 I.U/Kg/day i.m.) dissolved in sunflower oil on alternate days beginning from day 1 for a period of 5 weeks (35 days).
Group IV: Rotenone plus vitamin E treated
Animals in this group were given a combined treatment of rotenone (2 mg/kg body weight s.c.) daily and vitamin E (100 I.U/Kg/day i.m.) on alternate days for a period of five weeks (35 days).
Behavioral studies
Measurement of locomotor activity
Total locomotor activity (ambulations and rearing) was measured by using a computerized Actophotometer (IMCORP, India). An array of 16 infrared emitter/detector pairs measured animal activity along a single axis of motion, the digital data being displayed on the front panel meters as ambulatory movements. Rats were allowed to acclimatize to the observation chamber for a period of 2 minutes. The activity was monitored continuously for a period of 5 minutes. Locomotion was expressed in terms of total photo beam counts per 5 minutes per animal [21].
Catalepsy
The bar test was used for measuring catalepsy by the method of Costall and Naylor [22]. In the bar test, the rats were placed with both front paws on a horizontal bar which was 9 cm above and parallel from the base. The rats were placed with both front paws on the bar in a half rearing position; here they were timed with the stopwatch. When the animals removed one paw from the bar the stopwatch was stopped and the time noted. The maximum cutoff for bar test was fixed at 180 sec.
Neurochemical estimations
Biogenic amine, Dopamine was estimated by HPLC with electrochemical detector by the method of Church [23]. Waters standard system consisting of a high pressure isocratic pump, a 20 µl sample injector valve, C18 reverse phase column and electrochemical detector was used. Data was recorded and analyzed with the help of Empower software. Mobile phase consisting of 2% citric acid, 2% KHPO4, 1 mMEDTA, 1.2% MeOH, and 70 mg/ml of sodium octyl sulphate, pH of the mobile phase was adjusted to 3 with the help of HCl (6N). Electrochemical conditions for the experiment were +0.800 V, sensitivity ranges from 5~50 nA. Separation was carried out at a flow rate of 1 ml/min. Samples (20 µl) were injected manually.
Sample preparation
Fresh tissue samples from the mid brain region of rats were taken out and were homogenized in homogenising solution containing 0.1 M perchloric acid. 20% tissue homogenate was freshly prepared. Homogenate was centrifuged at 12,000×g for 5 min. The supernatant was further filtered through 0.25 micron nylon filters before injecting in the HPLC injection pump. Data was recorded and analyzed with the help of Empower software [21, 24].
Biochemical estimations
Determination of total glutathione
The estimation is done by the method of Zahler and Cleland [25]. The method is based on reduction of glutathione with dithioerytheritol and determination of the resulting monothiols at 412 nm with DTNB in the presence of arsenite.
Reduced glutathione estimation
GSH was estimated as the total non-protein sulphydryl groups by the method described by Moron et al. [26]. In this method 5, 5'-dithiobis-(2-nitrobenzoic acid) (DTNB) is reduced by the -SH groups of GSH to form one mole of 2-nitro-5-mercaptobenzoic acid per mole of -SH. The nitromercaptobenzoic acid anion released has an intense yellow color, which can be used to measure -SH groups at 412nm. Briefly, homogenates (10% , 100 µl) were immediately precipitated with 25 µl of 25% TCA and the precipitate was removed by centrifugation at 1,500×g for 10 min. 50 µl of the supernatant was added to 450 µl of sodium phosphate buffer (0.2 M, pH 8.0) to which was added 1ml of DTNB (0.6 mM prepared in phosphate buffer). The absorbance of the yellow color complex was read at 412 nm. GSH was used as a standard to calculate the content of GSH which is expressed as µmol of GSH/mg of protein.
Determination of oxidized glutathione
Oxidized glutathione was calculated by subtracting the value of glutathione reduced from total glutathione.
Lipid peroxidation assay
The assay for lipid peroxidation was performed according to the method of Wills [27]. Lipids mainly Poly Unsaturated fatty acids are highly susceptible to peroxidation by means of various oxidizing free radicals which are formed from various enzymatic and non-enzymatic reactions. Cycloperoxides are formed as a result of peroxidation reaction, which give MDA by cleavage. MDA forms a pink colored complex with Thiobarbituric acid whose absorbance can be read at 532 nm. The results were expressed as nmoles MDA/mg protein using molar extinction coefficient of MDA-thiobarbituric acid chromophore (1.56 *105 M-1 cm-1).
Superoxide dismutase activity
Superoxide dismutase (SOD) activity was assayed according to the method of Kono [28] wherein the reduction of nitrobluetetrazolium (NBT) was inhibited by SOD was measured at 560 nm. Briefly, the reaction was initiated by the addition of hydroxylamine hydrochloride to the reaction mixture containing NBT and PMS. The results were expressed as units/mg protein, where one unit of enzyme is defined as the amount of enzyme inhibiting the rate of a reaction by 50%.
Protein estimation
The protein content was measured according to the method of Lowry et al. [29].
Light microscopic examination
To assess histoarchitectural changes if any, small sections of hippocampus from each of the normal control and different treated animals were taken, washed with ice-cold 0.9% NaCl and were fixed in the buffered formalin (10%) for about 24~48 h. After the fixation, tissues were dehydrated in ascending grade of alcohol, embedded in wax following the standard technique [30]. 5~7 um thick paraffin sections were cut and then were subjected to hematoxylin-eosin staining as described by Humanson [31].
Statistical analysis
All values were expressed as mean±SD (n=6, per group). Data was analyzed using one way analysis of variance (ANOVA) followed by Newman-Keuls test for multiple pair wise comparisons between the various treated groups. Values with p<0.005 were considered to be statistically significant.
RESULTS
Effect of Vitamin E on body weights of rotenone treated animals
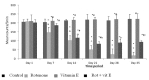
Fig. 1 depicts body weights of animals which in control group was found to be increased significantly (p<.001) at the end of day 35 whereas in rotenone treated animals (Group II) showed a gradual decrease in body weight during the course of treatment and significant decrease was recorded at the end of 5th week of rotenone exposure. However, in case of Vitamin E treatment group significant increase in body weight was recorded after 5th of rotenone exposure.
Effect of Vitamin E on rotenone induced motor dysfunction
Total locomotor activity
Significant (p<.001) decrease in total locomotor activity of rotenone treated animals was seen as compared to control animals at all intervals of time and the decline was highly (p<.001) significant decrease of 75% after 3rd week of rotenone exposure (Fig. 2). Co-administration of vitamin E along with rotenone significantly (p<.001) prevented the decrease in the total locomotor activity in rotenone treated animals which was found to increase by 54% at the end of 5th week (Fig. 2).
Catalepsy
Control animals depicted fall off time of a few seconds (Fig. 3) whereas animals treated with rotenone registered a significant increase in fall off time as compared to control at subsequent weeks . The effect was most prominent at 3 weeks of rotenone exposure with an increase of 85.95% in fall off time which further increased to 99.5% till the end of 5th week. Co-administration of vitamin E in rotenone treated animals significantly prevented the increase in fall off time at all intervals i.e the increase in falloff time which was 99.5% at the end of 5th week in rotenone treated animals was reduced to 63% with vitamin E co-treatment.
Effect of Vitamin E on neurotransmitter Dopamine (DA) level using (HPLC-ECD)
Chronic administration of rotenone resulted in significant decrease of 80% in the level of neurotransmitter Dopamine (p<0.001) (>50%) after 35 days of rotenone treatment and co-treatment of Vitamin E showed significant increase in Dopamine (p<0.05) level when compared to rotenone treated animals (Fig. 4).
Effect of Vitamin E on the biochemical parameters in rotenone infused animals
Glutathione system enzymes (Total Glutathione, GSH and GSSG)
Rotenone exposure significantly (p<.005) decreased the GSH levels by 47.36% and redox ratio in mid brain region of rotenone treated animals when compared to control as seen from (Table 1). Increase in the level of GSSG i.e oxidised glutathione could be related to increased conversion of GSSG from GSH. Rotenone plus Vitamin E treated animals showed significant increase of 29% in GSH level in rotenone treated animals as compared to rotenone treatment group.
Lipid peroxidation
MDA levels marker for lipid peroxidation was found to be increased significantly (p<.001) by 55% in mid brain region of rotenone treated animals as compared to control (Table 2). Vitamin E administration significantly prevented the increase (decreased by 64.95%) in amount of Lipid peroxidation as observed from lowered levels of MDA production in Vitamin E co-treatment group as compared to rotenone treated group (Table 2).
Superoxide dismutase (SOD)
Significant decrease of 41.46% in levels of SOD was observed in rotenone treated animals as compared to control (Table 2) after 35 days of rotenone exposure. However the level of SOD increased significantly (p<.001) in Vitamin E co-treatment group 54.5% as compared as compared to rotenone treated animals.
Histopathology
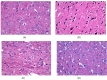
Histopathology of our samples showed that rotenone caused degeneration in the mid brain region of rats as neurons were under oxidative stress as can be seen from Fig. 5B. Darkly stained nucleus indicates hypoxic damage to neurons in rotenone treated animals. Also formation of dark dense granules lying on or close to the surface of neurons known as incrustations can be seen. Microvacuolation in which cytoplasm stains intensely and forms dark blue to mauve can be seen in rotenone treated animals can be seen at the end of 5th week as seen from Fig. 5B. However co-administration of Vitamin E showed a striking level of protection with no signs of adverse effects of the rotenone treatment in the mid brain Fig. 5D.
DISCUSSION
Parkinson's Disease, is the second most common neurodegenerative disorder with an incidence of around 14 per 100,000 population worldwide [32] and roughly 50,000 new cases arise every year. Epidemiological studies suggest that exposure to pesticide can increase the risk of PD [33]. At present, the etiopathology of PD is unknown. However, the selective oxidative stress in the SN is the earliest and the most broadly accepted hypothesis for the etiopathology of PD [34, 35]. This assumption was based on multiple observations in animal models of PD and also in human parkinsonian brains [36]. Consequently an, antioxidant therapy that can be administrated for a longer period of time, has minimal adverse effects and should slow down the progression of the disease has been suggested.
Rotenone, causes highly selective dopaminergic degeneration and alpha-synuclein aggregation in dopaminergic neurons, in vivo rodent model [8, 37] and its chronic subcutaneous exposure reproduced characteristics features of PD via excess generation of free radicals mediated oxidative stress. In the present study chronic administration of vitamin E demonstrated significant improvement in the neurobehavioral, oxidative as well as neurochemical parameters as caused by rotenone exposure. It has already been reported that Vitamin E (alpha-tocopherol) supplementation blocked rotenone-induced reductions in TH protein and TH immunohistochemical changes. A greater number of TH-positive neurons following one month of vitamin E pretreatment can be attributed to the neuroprotective effect of a high dose of vitamin E against 6-OHDA-induced toxicity that is well matched with the results of others [38, 39]. Moreover in clinical trials, it has been seen that the vitamin E therapy has retarded the progression of degenerative process in PD patients [40, 41]. Vitamin E given with rotenone attenuated the rotenone-induced oxidative stress [42] and exhibits a dose dependent effect in PD patients [14]. Also, it has been reported that the levels of glutathione and vitamin E increased in the brain of patients with PD as a compensatory mechanism to deal with oxidative stress [41-43].
The dose of (2 mg/kg) rotenone was well tolerated by animals as the mortality rate observed was quite low about 8%. Vitamin E at a dose of (100 I.U/kg/b.wt, i.m.) was co-administered with rotenone on alternate days during the course of treatment. The body weight of rotenone treated animals decreased gradually till 3rd week and there was a drastic decline in the body weight at the end of 5th week (Fig. 1). Similar results were obtained in our earlier study [24]. This decline in body weight of animals following rotenone exposure could be related to delay in gastric emptying occurred during rotenone intoxication [44]. Gastrointestinal dysfunction is the most common symptom of PD and the majority of symptoms, such as early satiety and weight loss, constipation, bloating, and dyspagia result from abnormal motility of the GI tract [45-47] whereas vitamin E modulates gastric motility and gastric lesion formation in rats [48-50].
At the end of 5th week, rotenone exposure caused a significant decline of (82%) in the level of neurotransmitter dopamine was observed in mid brain region (Fig. 4). The study results were further supported by earlier study reports showing decline in the neurotransmitter (DA) level following rotenone exposure [22]. significant and progressive decrease in total locomotor activity and increased rigidity (Catalepsy) was also observed which became significant at the end of 3rd week and declined substantially at the end of 5th week (Fig. 3). Biochemical oxidative stress markers reported a remarkable change following rotenone exposure. Decline in GSH level which is one of the earliest biochemical changes seen in PD [51, 52] and is believed to be the most robust and significant alteration in the antioxidant defense system was observed. Following rotenone exposure GSH level was found to decrease significantly (47%) at the en d of 5th week of rotenone exposure (Table 1, 2). Our observations were in line with the observations made in previous studies [24, 45, 53]. Similarly, a decrease in SOD activity was observed. A significant elevation in the level of MDA generation, an indicator of lipid peroxidation, was observed at the end of 3rd week which increased further at 5th week [54]. This relates to the findings that free radical generation and oxidative damage is involved in the neuronal abnormalities in PD [55-58]. The changes observed following rotenone exposure are in correlation with the manifestation of the PD symptoms.
However, Vitamin E treatment reduced LPO levels and brought them near to control levels as can be seen from (Table 1, 2). Lipid peroxidation is a self propagating process that will proceed until the substrate is consumed or termination occurs. Antioxidants like vitamin E (α-tocopherol), ascorbate, reduced glutathione) intercede by limiting the propagation process. The two broad outcomes to lipid peroxidation, viz., structural damage to membranes and generation of bioactive secondary products. Membrane damage derives from the generation of fragmented fatty acyl chains, lipid-lipid crosslinks, and lipid-protein crosslinks [59]. Since, vitamin E is lipid soluble, chain breaking antioxidant in biological membranes [59, 60] and forms the integral part of cellular membranes. It protects the biologiclal membranes against free radical damage by trapping the free radicals [61]. Vitamin E forms the first line of defence against lipid peroxidation as it prevents cell damage by binding to the free radicals and neutralising its unpaired electron forming a more stable intermediate structure that is converted to α-tocopherylquinone. Results from previous studies also reported that vitamin E, attenuates the effects of lipid peroxidation by trapping these free radicals [61-63]. With vitamin E supplementation the decreased levels of LPO resulted in lesser utilisation of glutathione and SOD which are touted as the first line of defence against free radical insult and thus decrease in GSH in rotenone plus vitamin E treatment group was much less. Treatment with vitamin E proved to be beneficial in replenishing the loss in GSH at 3rd week and 5th week. This is turn was reflected in SOD. Co-treatment with vitamin E was found to restore the SOD activity at 3rd week and at 5th week. Studies on synergistic effect of GSH and vitamin E suggest that vitamin E alone is more effective in decreasing LPO the main cause of oxidative stress of ethanol exposure [64].
Thus, the study demonstrates that vitamin E protects nigrostriatal dopaminergic neurons against degenerative effects induced by rotenone. Vitamin E by virtue of its free radical scavenging ability efficiently reduced rotenone induced lipid peroxidation and ROS generated oxidative stress. Also, with vitmain E supplementation there is lesser utilisation of GSH and SOD or we can say that vitamin E replaced the protective enzymes and mechanisms that are deficient in nigral neurons thus sparing the scavenging systems i.e SOD and GSH from the injurious effects of neurotoxins like rotenone and 6-OHDA [40-42]. Thus, providing protection to the antioxidant defense system in dopaminergic neurons hence, lesser DA-nergic neurodegeneration [65]. The motor dysfunction clearly correlates with the nigrostriatal dopaminergic cell loss and dopamine deficiency [24, 65, 66] as the neurons of DA-nergic cells project to the striatum and this leads to alterations in neuronal circuits within basal ganglia which are essential in regulating the various movements. Thus, supplementation with vitamin E showed significant improvement in Total locomoter activity and Catalepsy at the end of 3rd week which improved further at the end of 5th week.
Taken altogether, it is clear that rotenone at a chronic low dose is able to establish a PD model. Most of the changes were evident only after 3 weeks of rotenone exposure. Vitamin E being a free radical scavenger prevents the cell damage by binding to the free radicals and neutralizing its unpaired electron was able to prevent the damage to DNergic neurons caused by rotenone induced oxidative stress. However, no significant differences were observed between 3 weeks and 5 weeks of co-treatment of vitamin E with rotenone suggesting that several mechanisms other than oxidative stress could be involved during the progression of the disease. Histapathological analysis is in line with the recent reports on histopathological analysis in mid brain of rotenone treated animal [67]. This further supports the antioxidative role of vitamin E in rotenone induced PD.
CONCLUSION
Considering that antioxidant therapy is a major issue in PD, we propose that our findings reinforce the potential antioxidant role of vitamin E in the nigrostriatal system. From our data, we anticipate a beneficial role of this vitamin in the course of the disease can be used as a supplement to the existing therapeutic regimes. However, further studies are essential before approaching to any clinical implications.
Figures
Tables
Table 1. Effect of Vitamin E treatment on Glutathione system enzymes in mid brain of rotenone treated rats at day 21 and day 35
Data is mean±SEM of 6 animals. *p<0.05 significant when compared with control group. #p<0.05 significant when compared to rotenone treated animals.
Table 2. Effect of Vitamin E treatment on LPO and SOD activity in mid brain of rotenone treated rats at day 21 and day 35
Data is mean±SEM of 6 animals. *p<0.05 significant when compared with control group. #p<0.05 significant when compared to rotenone treated animals.
References
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link?. Exp Neurol 2005;193:279-290.
- Fahn S. An open trial of high-dosage antioxidants in early Parkinson's disease. Am J Clin Nutr 1991;53:380S-382S.
- Di Monte DA, Langston JW. In: Kettenmann H, Ransom BR. Neuroglia. New York, NY: Oxford University Press; 1995. p. 989-997, Chapter 65
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron 2003;39:889-909.
- Olanow CW, Schapira AH, Agid Y. Neuroprotection for Parkinson's disease: prospects and promises. Ann Neurol 2003;53:S1-S2.
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med 1998;339:1044-1053.
- Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, Crossman AR, Bioulac B, Brotchie JM, Gross CE. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Neurosci 2001;21:6853-6861.
- Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, Matsuno-Yagi A, Miller GW, Greenamyre JT. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson's disease. J Neurochem 2007;100:1469-1479.
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci 2003;23:10756-10764.
- Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 2002;136:317-324.
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 2000;3:1301-1306.
- Schmidt WJ, Alam M. Controversies on new animal models of Parkinson's disease pro and con: the rotenone model of Parkinson's disease (PD). J Neural Transm Suppl 2006;:273-276.
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem 2002;81:1285-1297.
- Amano S, Ohashi M, Kirihara M, Yang XH, Hazama F. Alpha-tocopherol protects against radical-induced injury in cultured neurons. Neurosci Lett 1994;170:55-58.
- Fujimoto S, Mizoi K, Yoshimoto T, Suzuki J. The protective effect of vitamin E on cerebral ischemia. Surg Neurol 1984;22:449-454.
- Ishihara L, Brayne C. A systematic review of nutritional risk factors of Parkinson's disease. Nutr Res Rev 2005;18:259-282.
- Yacoubian TA, Standaert DG. Targets for neuroprotection in Parkinson's disease. Biochim Biophys Acta 2009;1792:676-687.
- Butterfield DA, Castegna A, Drake J, Scapagnini G, Calabrese V. Vitamin E and neurodegenerative disorders associated with oxidative stress. Nutr Neurosci 2002;5:229-239.
- Peyser CE, Folstein M, Chase GA, Starkstein S, Brandt J, Cockrell JR, Bylsma F, Coyle JT, McHugh PR, Folstein SE. Trial of D-alpha-tocopherol in Huntington's disease. Am J Psychiatry 1995;152:1771-1775.
- Golbe LI, Farrell TM, Davis PH. Case-control study of early life dietary factors in Parkinson's disease. Arch Neurol 1988;45:1350-1353.
- Bishnoi M, Chopra K, Kulkarni SK. Involvement of adenosinergic receptor system in an animal model of tardive dyskinesia and associated behavioural, biochemical and neurochemical changes. Eur J Pharmacol 2006;552:55-66.
- Costall B, Naylor RJ. On catalepsy and catatonia and the predictability of the catalepsy test for neuroleptic activity. Psychopharmacologia 1974;34:233-241.
- Church WH. Column chromatography analysis of brain tissue: an advanced laboratory exercise for neuroscience majors. J Undergrad Neurosci Educ 2005;3:A36-A41.
- Nehru B, Verma R, Khanna P, Sharma SK. Behavioral alterations in rotenone model of Parkinson's disease: attenuation by co-treatment of centrophenoxine. Brain Res 2008;1201:122-127.
- Zahler WL, Cleland WW. A specific and sensitive assay for disulfides. J Biol Chem 1968;243:716-719.
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979;582:67-78.
- Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J 1966;99:667-676.
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 1978;186:189-195.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-275.
- Humason GL. Animal tissue techniques. San Francisco, CA: W. H. Freeman and Company, 1962; 1962. p. 130-132.
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders?. Neurology 2007;68:326-337.
- Richardson JR, Shalat SL, Buckley B, Winnik B, O'Suilleabhain P, Diaz-Arrastia R, Reisch J, German DC. Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol 2009;66:870-875.
- Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev 1997;25:335-358.
- Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology 1996;47:S161-S170.
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 1996;36:83-106.
- Alam M, Mayerhofer A, Schmidt WJ. The neurobehavioral changes induced by bilateral rotenone lesion in medial forebrain bundle of rats are reversed by L-DOPA. Behav Brain Res 2004;151:117-124.
- Cadet JL, Katz M, Jackson-Lewis V, Fahn S. Vitamin E attenuates the toxic effects of intrastriatal injection of 6-hydroxydopamine (6-OHDA) in rats: behavioral and biochemical evidence. Brain Res 1989;476:10-15.
- Perumal AS, Gopal VB, Tordzro WK, Cooper TB, Cadet JL. Vitamin E attenuates the toxic effects of 6-hydroxydopamine on free radical scavenging systems in rat brain. Brain Res Bull 1992;29:699-701.
- de Rijk MC, Breteler MM, den Breeijen JH, Launer LJ, Grobbee DE, van der Meché FG, Hofman A. Dietary antioxidants and Parkinson disease. The Rotterdam Study. Arch Neurol 1997;54:762-765.
- Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in Parkinson's disease. Prog Neurobiol 1996;48:1-19.
- Reiter RJ. Oxidative damage in the central nervous system: protection by melatonin. Prog Neurobiol 1998;56:359-384.
- Stoof JC, Vermeulen RJ, Van Royen EA, Drukarch B, Voorn P, Wolters EC, Groenewegen HJ. Dopaminergic systems and Parkinson's disease: some latest developments in pathogenetic, diagnostic and pharmacotherapeutic investigations. Neurosci Res Commun 1996;18:133-142.
- Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson's disease. Exp Neurol 2009;218:154-161.
- Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease: frequency and pathophysiology. Neurology 1992;42:726-732.
- Natale G, Pasquali L, Ruggieri S, Paparelli A, Fornai F. Parkinson's disease and the gut: a well known clinical association in need of an effective cure and explanation. Neurogastroenterol Motil 2008;20:741-749.
- Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol 2003;2:107-116.
- Garg MC, Bansal DD. Protective antioxidant effect of vitamins C and E in streptozotocin induced diabetic rats. Indian J Exp Biol 2000;38:101-104.
- Perkin GD, Murray-Lyon I. Neurology and the gastrointestinal system. J Neurol Neurosurg Psychiatry 1998;65:291-300.
- Ibrahim IA, Kamisah Y, Nafeeza MI, Nur Azlina MF. Modulation of gastric motility and gastric lesion formation in stressed rats given enteral supplementation of palm vitamine E and α-tocopherol. Int Med J 2011;18:47-52.
- Martin HL, Teismann P. Glutathione--a review on its role and significance in Parkinson's disease. FASEB J 2009;23:3263-3272.
- Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. J Neural Transm 1997;104:661-677.
- Kaur H, Chauhan S, Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson's disease. Neurochem Res 2011;36:1435-1443.
- Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem 1989;52:381-389.
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Parkinsonism in HIV dementia. J Neural Transm 2002;109:767-775.
- Adams JD, Odunze IN. Oxygen free radicals and Parkinson's disease. Free Radic Biol Med 1991;10:161-169.
- Singh C, Ahmad I, Kumar A. Pesticides and metals induced Parkinson's disease: involvement of free radicals and oxidative stress. Cell Mol Biol (Noisy-le-grand) 2007;53:19-28.
- Farber JL. In: Craighead JE. Pathology of environmental and occupational disease. St. Louis, MO: Mosby, 1995; 1995. p. 287-302.
- Burton GW, Traber MG. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr 1990;10:357-382.
- Muller DP, Lloyd JK, Wolff OH. Vitamin E and neurological function. Lancet 1983;1:225-228.
- Willson RL. Free radical protection: why vitamin E, not vitamin C, beta-carotene or glutathione?. Ciba Found Symp 1983;101:19-44.
- Halliwell B, Gutteridge JM. Oxygen radicals and the nervous system. Trends Neurosci 1985;8:22-26.
- Fariss MW, Zhang JG. Vitamin E therapy in Parkinson's disease. Toxicology 2003;189:129-146.
- Gupta A, Singh S, Jamal F, Nath S, Mehrotra S, Sharma B. Synergistic effects of glutathione and vitamin E on ROS mediated ethanol toxicity in isolated rat hepatocytes. Asian J Biochem 2011;6:347-356.
- Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res 2005;134:109-118.
- Selby G. In: Stern GM. Parkinson's disease. Baltimore, MD: The Johns Hopkins University Press, 1990; 1990. p. 333-388.
- Pearse AGE. Histochemistry: theoretical and applied. 3rd ed. London: Churchill Livingstone, 1968; 1968. p. 660.
- Abdel Moneim AE, Dkhil MA, Al-Quraishy S. The potential role of Portulaca oleracea as a neuroprotective agent in rotenone-induced neurotoxicity and apoptosis in the brain of rats. Pestic Biochem Physiol 2013;105:203-212.