Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2013; 22(4): 258-267
Published online December 30, 2013
https://doi.org/10.5607/en.2013.22.4.258
© The Korean Society for Brain and Neural Sciences
Curcumin Can Prevent the Changes in Cerebellar Structure and Function Induced by Sodium Metabisulfite in Rat
Ali Noorafshan1,2, Ali Rashidiani-Rashidabadi2, Saied Karbalay-Doust1,2*, Aghdas Poostpasand2, Mohammad-Amin Abdollahifar2 and Reza Asadi-Golshan2
1Histomorphometry and Stereology Research Centre, Shiraz University of Medical Sciences,2Anatomy Department, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Fars 71348-45794, Iran
Correspondence to: *To whom correspondence should be addressed.
TEL: 98-711-2304372, FAX: 98-711-2304372
e-mail: karbalas@sums.ac.ir
Sulfites are used as anti-microbial and anti-oxidant agents in the food and pharmaceutical industries. Curcumin, a flavonoid, is an Asian spice that shows neuroprotective activities. The current study aimed to stereologically assess the rats' cerebellar cortex and rotarod performance following sulfite exposure and determine the possible neuroprotective potential of curcumin. The rats were divided into five groups: distilled water, olive oil, curcumin (100 mg/kg/day), sodium metabisulfite (25 mg/kg/day), and sodium metabisulfite+curcumin. At 56 days after treatment, rotarod performance was tested, and then the cerebellum was removed for stereological analysis. The study results revealed 31%, 36%, 19% and 24% decrease in the total volume of the cerebellum, cortex, the total number of the Purkinje cells and length of the nerve fibers in the cortex per Purkinje, respectively in the sodium metabisulfite-treated rats compared to the distilled water group (p<0.01). The pre-trained animals on the rotarod apparatus were tested first on the fixed speed rotarod protocol followed by the accelerating rotarod protocol two days later. The results showed a significant decrease in the latency to fall in both test in sulfite-treated rats. The sulfite effects on the structural parameters and rotarod performance were significantly protected by the concomitant curcumin treatment (p<0.001). Sulfite can induce structural and functional changes in the rats' cerebellum and concomitant curcumin prescription plays a neuroprotective role.
Keywords: cerebellum, sulfite, curcumin, rat, histology
Sulfiting agents are food preservatives composed of potassium or sodium bisulfite or potassium metabisulfite. Sulfiting agents are used in the processing of some drinking, baked goods, soup mixes, and some imported sea foods and by restaurants to ration a "fresh" appearance to salad fruits and vegetables. These compounds act as antimicrobials and antioxidants [1]. Sulfite can cause cellular toxicity by reacting with a variety of humoral and cellular components [2]. Catabolism of amino acids and other compounds that contain sulfite can naturally generate large quantities of sulfite in the body [2].
However, sulfite oxidase is an enzyme (located in the mitochondria) which oxidizes sulfite to sulfate. This protects the cells from the toxic effects of sulfite [3]. If sulfite oxidase is deficient as in a hereditary disorder, this detoxification process cannot occur. Finally, this leads to neurological abnormalities, such as decrease in brain growth and mental retardation [3]. Activity of the enzyme is low in the brain, spleen, and testis [3]. Therefore, the chance of sulfite toxicity of neurons is high. It has been shown that sulfite can induce toxic effects on neurons. For example, exposure to sulfite reduced the hippocampal neuron number in rats [4]. In addition, sulfite treatment increased the excitability of the spinal reflexes [5]. It has also been reported in an in vitro study that sulfite and peroxynitrite anion induce toxic effects on neuronal cell lines [6].
Therefore, it is necessary to design a study in order to evaluate the effects of sodium bisulfite on different functional activities of different brain regions. Therefore, at the first step of the present study, the cerebellar cortex structure and the rotarod performance is tested after treatment of sulfite in an animal model. The second step of the study involves introducing a compound that could be added to the foods and be able to protect the motor coordination after sulfite consumption. Curcumin is a yellow Indian spice that has useful pharmacological effects, such as antioxidant, antiinflammatory, antiapoptotic, anticancer, and anti-infectious effects [7]. Up to now, many neuroprotective effects of curcumin have been shown. In addition, our previous studies have shown that curcumin displays protective effects on the sciatic nerve and dorsal root ganglion following crush in rats [8]. It has also been shown that curcumin has protective effects against neural disorders including diabetic neuropathy and cerebral ischemia [7]. The rotarod test is an instrumental technique for assessment of motor function in experimental animals that was described to evaluate lesion and drug effects on the motor coordination of the rodents due to damage of the basal ganglia and cerebellum [9]. In the present study, the rats were tested in two different protocols, including incremental fixed speed and accelerating speed after prescription of sulfite and curcumin [9]. The present research tries to answer the following questions:
How much does the cerebellar, cortical, and white matter volume change after sulfite-treatment in rats? Will the number of Purkinje cells change after sulfite-treatment in rats? Will the nerve fiber length change after sulfite-treatment in rats? Will the rotarod performance is impaired after sulfite treatment? Can curcumin prevent the possible changes after sulfite-treatment in rats?
MATERIALS AND METHODS
Fifty Sprague-Dawley male rats (weight, 250 to 280 g) were sampled from the laboratory animal center of Shiraz University of Medical Sciences, Shiraz, Iran. All the procedures were performed under the supervision of the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran and the animals were kept under standard condition. The ethics committee of the university agreed with the animal experiment under approval No 91-6070. The animals were randomly divided into five groups each including ten rats. Six rats in each group underwent the stereological study but all ten rats evaluated in rotarod test. The first and the second groups received distilled water and olive oil, respectively as the vehicles for sodium metabisulfite and curcumin. Besides, the third and fourth groups received sodium metabisulfite (25 mg/kg/day) and curcumin (100 mg/kg/day), respectively [4, 8, 10]. Finally, the fifth group received both sodium metabisulfite and curcumin in doses described above. All the administrations were done orally for 56 days. According to the studies of Akdogan et al. [4] and Ribera et al. [10] the time point was adopted. The study of Ribera et al. 2001 was performed on the rats for 28 (subacute) and 85 (subchronic) days of dietary contact [4, 10]. The time point adopted here (56 days) was selected as the average time point which was considered for subacute and subchronic toxicities. The animals were housed in plastic cages under a 12 h/12 h Light/Dark cycle with room temperature of 22±2℃.
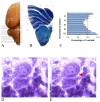
At the end of the 56th day, all the animals were sacrificed and their brains were dissected immediately. Then, the right cerebellar hemisphere was separated. The selected hemispheres were processed and serial coronal sections of 26-mm thickness were obtained and stained with cresyl violet. Cresyl-violet is used to demonstrate the nissl substance in the neurons and cell nuclei. It can be seen from Figure 1 that the cortex and white matter are distinguishable. Overall, 10 to 12 sections were sampled through systematic uniform random sampling. To locate the cerebellum regions, the rat brain atlas by Paxinos and Watson [11] was applied. Using a projecting microscope, the total volumes of the cerebellum, cortex, and white matter were estimated at the final magnification of 25× using Cavalieri's principle [12-14] (Fig. 1A, B). In doing so, the product of the areas and the distances between the sampled sections (
A computer connected to a Nikon E200 microscope (Nikon, Japan), ×60 oil immersion objective lens with a high numerical aperture (NA: 1.4), and an optical disector were used in order to estimate the total number of the Purkinje cells. The microscopic fields were sampled by moving the microscope stage in an equal interval using a stage micrometer and systematic uniform random sampling [12-14]. The movement of the microscope stage in the Z-axis was measured using a microcator (MT12, Heidenhain, Germany) attached to the stage [15]. Briefly, an unbiased counting frame with inclusion (right and upper) and exclusion (left and lower) borders was superimposed on the images of the sections viewed on the monitor. To calculate the suitable guard zone and the height of the disector (
The total number of the Purkinje cells was estimated by multiplying the numerical density (Nv) by the total volume of the cerebellar hemisphere. 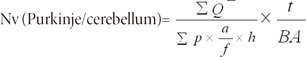
where "Σ
The CE for the estimate of the volume using point counting is the function of the noise effect and systematic random sampling variance for the sums of the estimated areas. Since the cross-sectional areas of the region of interest were estimated by point counting, CE (V) was calculated by the following formula [16-18]:
Where "b" and "a" represented the mean section boundary length and mean sectional area, respectively. The CE for the estimate of the total Purkinje cell number, CE(N), was calculated using CE(V) and CE(Nv) as follows:
The data are shown in Table 1.
Vertical uniform random sectioning is necessary for length estimation [18, 19]. Briefly, 9~10 cylinders were punched out using a trocar with diameter of 1 mm vertical to the pial surface of the cortex of the cerebellum (Fig. 2A). The cylinders were randomly rotated along their vertical axis and embedded in one paraffin block. Then, 100 µm thickness slabs were obtained using a microtome and mounted on the slide (Fig. 2B). The dendritic tree of the purkinje cells, parallel fibers of the granular cells, process of the basket, and stellate cells are present in the molecular layer of the cortex. The mean length of the nerve fibers per purkinje was calculated using the following formula [18, 19]:
A fixed slab height of T (here 100 µm) was scanned inside the slice thickness using a microscope (Nikon E-200) equipped with an objective lens 100× and numerical aperture of 1.4 connected to a computer. Then, the number (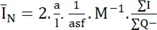
Where 
Briefly, we pre-trained the animals on the rotarod apparatus. In this study, after initial habituation, the rats were tested first on the fixed speed rotarod protocol and, two days later, on the accelerating rotarod protocol [9]. On the testing day, according to the fixed speed protocol, the animals were tested at 7 different speeds, including 12, 16, 19, 21, 24, 26, 28, and 38 rpm for a maximum of 60 s at each speed. The rats were tested for three times at each speed with an interval of 20 min between each trial. Latency of each rat to fall from the rotarod was registered automatically with stop watches connected to the detectors [17]. According to the accelerating protocol, the animals were placed on the rod whose rotation speed steadily increased from 4 to 40 rpm during a 300 s period. The latency to fall was recorded as described previously [9].
The stereological data were analyzed using Kruskall-Wallis and Mann-Whitney's U-tests. Besides, p<0.05 was considered as statistically significant. For the rotarod performance, statistical analyses were undertaken by repeated measures two-way ANOVA.
The results of the stereological estimations are present as the dot plots (Fig. 3) which is a standard form of presentation of the data obtained using stereological methods. The total volume, number and length were shown in the rats of different groups. Each dot represents the parameter of an animal and the small horizontal bar indicates the average of the data. In this form of presentation the distribution of the data around the mean can also be observed.
The study results revealed ~31% and 36% decrease in total volume of the cerebellum and cortex, respectively in the sodium metabisulfite-treated rats compared to the distilled water group (p<0.01). However, no significant change was observed in the white matter volume. The volume of the cerebellum and its cortex did not change in the sulfite+curcumin-treated animals in comparison to the distilled water-treated rats (Fig. 3A, B, C). This finding indicates the protective effects of curcumin.
The findings of the study revealed ~19% decrease in the total number of the Purkinje cells in the sodium metabisulfite-treated rats compared to the distilled water group (p<0.01). The number of the Purkinje cells did not show any change in the sulfite+curcumin-treated animals in comparison to the distilled water-treated rats (Fig. 3D).
The mean total length of the nerve fibers per Purkinje cell was estimated in different groups (Fig. 3). The study findings revealed that the length of the nerve fibers in the cerebellar cortex was decreased by ~24% in the sulfite-treated animals in comparison to the distilled water group (p<0.01). However, no significant reduction was detected in their length in the sulfite+curcumin-treated rats in comparison to the sulfite-treated animals (Fig. 3E). The results also showed that curcumin or its vehicle (olive oil) did not affect the length of the fibers.
Fig. 4A summarizes the results of the protocol. The results of this experiment demonstrated that administration of sodium metabisulfite impaired (p<0.001) the rotarod performance that was significantly different from the other groups. In this experiment, all the animals gavaged with sodium metabisulfite showed a significant decrease in the latency to fall compared to the other groups.
In contrast, no significant difference was found between the other groups. In fact, their performance was nearly stable at below 26 rpm speeds, leading to mean latencies to fall of about 45~60 s and the latency to fall was just slightly decreased at higher speeds; i.e., 26 to 38 rpm (Fig. 4A).
In addition, the present results showed that the sodium metabisulfite effect was significantly recovered in the rats treated with sulfite+curcumin. This means that curcumin could protect the sodium metabisulfite-induced impairment of the rotarod performance.
The results of accelerating protocol are presented in Figure 4B. A significant reduction in rotarod performance was observed in sodium metabisulfite group (p<0.001). In this protocol, the performance of the other groups did not significantly differ from the distilled water group and their mean latency to fall was approximately 120~150 s. Furthermore, the latency to fall was significantly increased in the rats treated with sulfite+curcumin compared to the sodium metabisulfite group.
Stereology is a popular technique to estimate the quantitative data of three-dimensional materials. It has helped to detect tissue structural changes in histo-pathological conditions. Considering the role of cerebellum in learning, cognition, and control of motor functions [20], evaluation of stereological parameters, including cerebellar tissues volume and Purkinje cell number, may be useful to uncover the histological changes in cerebellar disorders. The first step of the present work elucidated the effects of sulfite on the total volume of the cerebellum, cortex, and white matter, the total number of Purkinje cells and fiber length per purkinje in the sulfite-treated group. Our results indicated that all these parameters, except for white matter, were significantly decreased in the sulfite-induced group compared to the control group. The reduction in the total volume of the cerebellum and cortex can be attributed to the loss of Purkinje cells. These findings are consistent with those of a previous study showing the toxic effects of sulfite on the rats' mesencephalic cell line. These observations are in agreement with the previous studies showing the sulfite toxicity on neurons. For example, Akdogan et al. [4] showed loss of pyramidal neurons in the subdivisions of the rat hippocampus caused by sulfite treatment. The rotarod apparatus has been used to evaluate changes in motor function caused by some pharmacological agents, such as tranquilisers and antidepressants and antianxiety compounds [21, 22]. In the present study, the rotarod test was used to evaluate the effect of sodium metabisulfite on motor coordination. The results showed impairment of the performance of the sulfite-treated rats in rotarod after 56 days. Furthermore, our previous work demonstrated that sulfite administration impaired learning and memory in rats [23].
It has been shown that neurotoxic effect of peroxynitrite radicals is potentiated by sulfite [6]. Although the mechanism of sulfite toxicity on the central nervous system is not completely understood, sulfur- and oxygen-centered free radicals may be responsible for this effect of sulfite [24]. Sulfite metabolism generates an intermediate product called sulfur trioxide radical that may act in some toxic effects of sulfite, including increment of lipid peroxidation, impairment of DNA synthesis, and destruction of amino acids [25]. It has also been indicated that the rats which possess normal sulfite oxidase enzyme activity can tolerate large amounts of sulfite and do not show toxicity [26]. It can be proposed that the harmful effects of sulfite can be attributed to cysteine-S-sulfate as a brain damaging metabolite which acts in sulfite oxidase enzyme deficiency. The human body does not have Cysteine-S-sulfate naturally [27]. It was found that rats have more ability in sulfite-oxidizing action compared to humans. It has also been reported that 750 mmol sulfite per kilogram body weight per day, equivalent to 48 g sulfite per kilogram body weight per day, is oxidized by sulfite oxidase enzyme in rats [28]. Moreover, some researchers have mentioned that various individuals have polymorphonuclear leukocytes with considerable variation in sulfite oxidase enzyme activity that is in agreement with the proposed polymorphic distribution of sulfite oxidase enzyme in humans [24-28]. Our results are confirmed by much evidence mentioned above about the detrimental effects of sulfite.
The doses of the sodium metabisulfite were prescribed according to the previous studies. Earlier studies have reported the acceptable daily intake of 163 mg/day by World Health Organization (WHO, 1994) from foods and drink in a single day or meal [2, 29, 30]. The acceptable daily intake of 0~0.7 mg/kg was assigned to sulfur dioxide and to sulfur dioxide equivalents arising from Na2S2O5. Therefore, it should be noted that the mean per capita of sulfite intake limit from food and beverages is estimated as 19 mg sulfur dioxide equivalents per day. This level is reported to be 163 mg/kg sulfur dioxide equivalents in the 99th percentile of the population [2, 29, 30]. However no one knows correctly how much sulfite is ingested by the general population because it depends on dietary regime.
The dose of curcumin was selected according to our earlier results. Our previous study showed that the appropriate dose for the curcumin with no side effects on the liver, kidney and blood levels of aspartate aminotransferase, alanine aminotransferase, urea nitrogen and creatinine was 100 mg/kg/day [7, 31].
Another goal of the current study was to assess the possible neuroprotective effect of curcumin. Our findings indicated that damaging sulfite effects were significantly reversed following curcumin administration in the fifth treatment group. In addition, curcumin treatment prevented the sulfite induced cerebellar damage as shown in Fig. 3 with no significant changes in the cerebellar volume and Purkinje cells number in the fifth group compared to the control group (distilled water). This observation shows the neuroprotective action of curcumin against sulfite. These findings are supported by our previous study showing the neuroprotective effect of curcumin on the dorsal root ganglion structure after sciatic nerve crush in rats. We also observed the functional and structural recovery by curcumin [8]. Another study has shown that curcumin has neuroprotective and anti-apoptotic effects on neurons following spinal cord injury [7]. Furthermore, other studies have reported that the regulation of the inflammatory cytokines, such as interleukin-6, tumor necrosis factor alpha, and cyclo-oxygenase-2, is modulated by curcumin. Therefore, curcumin shows anti-inflammatory effects [7]. Smith et al. [32] showed that the functional recovery after peripheral nervous system injury is prevented by early inflammatory events. Moreover, it was reported that neuroinflammation, excitotoxicity, cholinergic dysfunction, and oxidative stress in the cerebral cortex and hippocampus of okadaic acid treated mice were reduced as a result of curcumin administration. Also, memory function in both Morris water maze and passive avoidance tests was improved by curcumin administration in the mice with okadaic acid induced memory impairment [33]. In addition, studies have suggested that curcumin can improve the impaired memory of rats with chronic alcohol consumption [34]. It has been suggested that curcumin can modulate the release of serotonin and dopamine as an antidepressant. Moreover, the amount of neurotrophic factors, including brain derived neurotrophic factor, is elevated by curcumin [35]. Homocysteine neurotoxicity in the hippocampus of rats is inhibited by curcumin. The mechanism of protection appears to involve prevention of the formation of reactive oxygen species by curcumin in the rat's brain [36]. Curcumin is able to normalize the alterations caused by streptozotocin treatment in the cerebellum of rats [37]. Additionally, the findings of our previous study showed that curcumin had protective effects on the behavioral changes in chronic variable stress-induced rats [38]. Overall, the findings of the present study demonstrating the neuroprotective effect of curcumin are corroborated by the above-mentioned evidence.
Conclusion. Sulfite can induce changes in cerebellar cortex structure and functional changes in the rats' cerebellum and concomitant curcumin prescription plays a neuroprotective role.
Table 1. Coefficients of error (CE) for the total volume of the cerebellum, number as well as numerical density of the Purkinje cell in the cerebellum
- Lester MR. Sulfite sensitivity: significance in human health. J Am Coll Nutr 1995;14:229-232.
- Taylor SL, Higley NA, Bush RK. Sulfites in foods: uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity. Adv Food Res 1986;30:1-76.
- Mudd SH, Irreverre F, Laster L. Sulfite oxidase deficiency in man: demonstration of the enzymatic defect. Science 1967;156:1599-1602.
- Akdogan I, Kocamaz E, Kucukatay V, Yonguc NG, Ozdemir MB, Murk W. Hippocampal neuron number loss in rats exposed to ingested sulfite. Toxicol Ind Health 2011;27:771-778.
- Küçükatay V, Genç O, Kocamaz E, Emmungil G, Erken H, Bagci H. Spinal reflexes in normal and sulfite oxidase deficient rats: effect of sulfite exposure. Toxicol Ind Health 2008;24:147-153.
- Reist M, Marshall KA, Jenner P, Halliwell B. Toxic effects of sulphite in combination with peroxynitrite on neuronal cells. J Neurochem 1998;71:2431-2438.
- Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des 2013;19:2032-2046.
- Noorafshan A, Omidi A, Karbalay-Doust S. Curcumin protects the dorsal root ganglion and sciatic nerve after crush in rat. Pathol Res Pract 2011;207:577-582.
- Monville C, Torres EM, Dunnett SB. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods 2006;158:219-223.
- Ribera D, Jonker D, Narbonne JF, O'Brien J, Antignac E. Absence of adverse effects of sodium metabisulphite in manufactured biscuits: results of subacute (28-days) and subchronic (85-days) feeding studies in rats. Food Addit Contam 2001;18:103-114.
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. San Diego, CA: Academic Press, 2006.
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 1988;96:379-394.
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 1988;96:857-881.
- Kristiansen SL, Nyengaard JR. Digital stereology in neuropathology. APMIS 2012;120:327-340.
- von Bartheld CS. Distribution of particles in the z-axis of tissue sections: relevance for counting methods. Neuroquantology 2012;10:66-75.
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc 1987;147:229-263.
- Braendgaard H, Evans SM, Howard CV, Gundersen HJ. The total number of neurons in the human neocortex unbiasedly estimated using optical disectors. J Microsc 1990;157:285-304.
- Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc 1986;142:259-276.
- Howard CV, Cruz-Orive LM, Yaegashi H. Estimating neuron dendritic length in 3D from total vertical projections and from vertical slices. Acta Neurol Scand Suppl 1992;137:14-19.
- Akosman MS, Gocmen-Mas N, Karabekir HS. Estimation of Purkinje cell quantification and volumetry in the cerebellum using a stereological technique. Folia Morphol (Warsz) 2011;70:240-244.
- Herr F, Stewart J, Charest MP. Tranquilizers and antidepressants: a pharmacological comparison. Arch Int Pharmacodyn Ther 1961;134:328-342.
- Gluckman MI. Pharmacology of oxazepam (Serax), a new anti-anxiety agent. Curr Ther Res Clin Exp 1965;7:721-740.
- Noorafshan A, Asadi-Golshan R, Karbalay-Doust S, Abdollahifar MA, Rashidiani-Rashidabadi A. Curcumin, the main part of turmeric, prevents learning and memory changes induced by sodium metabisulfite, a preservative agent, in rats. Exp Neurobiol 2013;22:23-30.
- Abedinzadeh Z. Sulfur-centered reactive intermediates derived from the oxidation of sulfur compounds of biological interest. Can J Physiol Pharmacol 2001;79:166-170.
- Niknahad H, O'Brien PJ. Mechanism of sulfite cytotoxicity in isolated rat hepatocytes. Chem Biol Interact 2008;174:147-154.
- Küçükatay V, Savcioğlu F, Hacioğlu G, Yargiçoğlu P, Ağar A. Effect of sulfite on cognitive function in normal and sulfite oxidase deficient rats. Neurotoxicol Teratol 2005;27:47-54.
- Olney JW, Misra CH, de Gubareff T. Cysteine-S-sulfate: brain damaging metabolite in sulfite oxidase deficiency. J Neuropathol Exp Neurol 1975;34:167-177.
- Cohen HJ, Drew RT, Johnson JL, Rajagopalan KV. Molecular basis of the biological function of molybdenum: the relationship between sulfite oxidase and the acute toxicity of bisulfite and SO2. Proc Natl Acad Sci U S A 1973;70:3655-3659.
- Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. A critical review. CRC Crit Rev Toxicol 1987;17:185-214.
- Ercan S, Basaranlar G, Gungor NE, Kencebay C, Sahin P, Celik-Ozenci C, Derin N. Ghrelin inhibits sodium metabisulfite induced oxidative stress and apoptosis in rat gastric mucosa. Food Chem Toxicol 2013;56:154-161.
- Kheradpezhouh E, Panjehshahin MR, Miri R, Javidnia K, Noorafshan A, Monabati A, Dehpour AR. Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N-acetyl cysteine. Eur J Pharmacol 2010;628:274-281.
- Smith D, Tweed C, Fernyhough P, Glazner GW. Nuclear factor-kappaB activation in axons and Schwann cells in experimental sciatic nerve injury and its role in modulating axon regeneration: studies with etanercept. J Neuropathol Exp Neurol 2009;68:691-700.
- Rajasekar N, Dwivedi S, Tota SK, Kamat PK, Hanif K, Nath C, Shukla R. Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. Eur J Pharmacol 2013;715:381-394.
- Tiwari V, Chopra K. Protective effect of curcumin against chronic alcohol-induced cognitive deficits and neuroinflammation in the adult rat brain. Neuroscience 2013;244:147-158.
- Wang R, Li YB, Li YH, Xu Y, Wu HL, Li XJ. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res 2008;1210:84-91.
- Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH, Kazeminejad B. Neuroprotective effects of the polyphenolic antioxidant agent, Curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat. Pharmacol Biochem Behav 2010;96:378-385.
- Peeyush KT, Gireesh G, Jobin M, Paulose CS. Neuroprotective role of curcumin in the cerebellum of streptozotocin-induced diabetic rats. Life Sci 2009;85:704-710.
- Noorafshan A, Abdollahifar MA, Karbalay-Doust S, Asadi-Golshan R, Rashidian-Rashidabadi A. Protective effects of curcumin and sertraline on the behavioral changes in chronic variable stress-induced rats. Exp Neurobiol 2013;22:96-106.