Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2015; 24(4): 358-365
Published online December 30, 2015
https://doi.org/10.5607/en.2015.24.4.358
© The Korean Society for Brain and Neural Sciences
The Effect of Donor-Dependent Administration of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells following Focal Cerebral Ischemia in Rats
Hyung Woo Park1,2,3, Jong Wook Chang5, Yoon Sun Yang4, Wonil Oh4, Jae Ha Hwang1,2,3, Dong Gyu Kim1,2,3 and Sun Ha Paek1,2,3*
1Department of Neurosurgery, 2Cancer Research Institute, 3Ischemic/Hypoxic Disease Institute,
Seoul National University College of Medicine, Seoul 03082,
4Biomedical Research Institute, MEDIPOST Co., Ltd, Seoul 13494, 5Stem Cell & Regenerative Medicine Center,
Research Institute for Future Medicine, Samsung Medical Center, Seoul 06351, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-2072-3993, FAX: 82-2-744-8459
e-mail: paeksh@snu.ac.kr
Abstract
Stroke is an ischemic disease caused by clotted vessel-induced cell damage. It is characterized by high morbidity and mortality and is typically treated with a tissue plasminogen activator (tPA). However, this therapy is limited by temporal constraints. Recently, several studies have focused on cell therapy as an alternative treatment. Most researches have used fixed donor cell administration, and hence, the effect of donor-dependent cell administration is unknown. In this study, we administered 3 types of donor-derived human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) in the ischemic boundary zone of the ischemic stroke rat model. We then performed functional and pathological characterization using rotarod, the limb placement test, and immunofluorescent staining. We observed a significant decrease in neuron number, and notable stroke-like motor dysfunction, as assessed by the rotarod test (~40% decrease in time) and the limb placement test (4.5 point increase) in control rats with ischemic stroke. The neurobehavioral performance of the rats with ischemic stroke that were treated with hUCB-MSCs was significantly better than that of rats in the vehicle-injected control group. Regardless of which donor cells were used, hUCB-MSC transplantation resulted in an accumulation of neuronal progenitor cells, and angiogenic and tissue repair factors in the ischemic boundary zone. The neurogenic and angiogenic profiles of the 3 types of hUCB-MSCs were very similar. Our results suggest that intraparenchymal administration of hUCB-MSCs results in significant therapeutic effects in the ischemic brain regardless of the type of donor.
Keywords: hUCB-MSCs, Ischemia, Angiogenesis, Neurogenesis, Donor-dependent cell therapy
INTRODUCTION
Ischemic brain injury is a major cause of morbidity and mortality. Over 700,000 strokes occur each year in the United States, with a mortality rate of 27%. There are approximately 3 million stroke survivors with varying degrees of disability [1]. In recent years, the use of mesenchymal stem cells (MSCs) has received attention as a new alternative for treating this disease. MSCs exist in almost all tissues, including bone marrow, muscle, fat, hair follicles, tooth root, placenta, brain periosteum, dermis, perichondrium, umbilical cord, Wharton's jelly, lung, liver and spleen [2]. These stem cells were identified by their potential to undergo induced differentiation into several different mesenchymal lineages, such as bone, cartilage, adipose tissue, muscle, and tendon [3]. Recently, human umbilical cord blood-derived stem cells (hUCB-MSCs) have begun to show promise as a cell-based therapy. To date, no morphological differences between bone marrow-derived mesenchymal stem cells (BM-MSCs) and hUCB-MSCs have been reported [4,5,6].
Researchers have reported that gradual exposure to MSCs at a lesion site resulted in accumulation of various therapeutic proteins that were secreted and reacted with the microenvironment [7]. Therefore, many different proteins secreted by MSCs influenced paracrine actions for recovery of the lesion site. The therapeutic proteins secreted by MSCs included growth factors, cytokines, extracellular matrix proteins, and antioxidants. When the paracrine effect of MSCs was assessed in various disease models, there were similar therapeutic effects through the actions of the proteins mentioned above [7]. Although the general characteristics of MSCs include stemness, tropism, differentiation, motivation, and a therapeutic paracrine effect, it is possible that the origin of the donor cells (e.g., hUCB-MSCs from a pregnant mother) may influence their therapeutic effects.
HUCB (human umbilical cord blood) cells are likely to be used for transplantation into allogeneic recipients. HUCB cell banking may ensure more popular autologous transplants and may become more common in the future. However, HUCB collection can only be performed at certain time points (e.g., during birth), and the number of stem cells extracted from one donor is very limited. Importantly, treatments with MSCs should be profiled in the context of element-growth factor and cytokines [8,9]. The expression of these elements may differ depending on the origin of the cells or the culture process [10,11].
We sought to profile different donor cells and their stem cell properties as well as their therapeutic effects in animal disease models. Many studies have reported dominant cytokine expression in hUCB-MSCs. When hUCB-MSCs were used in an
MATERIALS AND METHODS
Adult male Sprague-Dawley rats (260~300 g; Orient bio, Korea) were housed in an animal care facility under a 12 h light/dark cycle, with food and water available
Rats were anesthetized by an intramuscular injection of Rompun (10 mg/kg of xylazine hydrochloride, Bayer, Leverkusen, Germany) and Zoletil 50 (75 mg/kg of zolazepam and tiletamine, Virbac, Carros, France). Rectal temperature was maintained at 37℃ using a thermistor-controlled heating blanket. Transient MCAO was induced using an intraluminal vascular occlusion method, as previously described [12]. Briefly, a 2 cm incision was made at the center of the neck; the left common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were exposed under an operating microscope. A 4/0 monofilament nylon thread whose tip was rounded by heating was advanced into the lumen of the ICA until it blocked the origin of the MCA. One and half hours after MCAO, animals were re-anesthetized for reperfusion, which was performed by suture withdrawal. After recovery from anesthesia, animals were allowed free access to food and water. After surgery, 28 of the 48 animals survived; consequently, a total of 28 rats were randomly assigned to the control and experimental groups.
This study was approved by the Institutional Review Board of MEDIPOST Co., Ltd. UCB-MSCs were separated and maintained as described previously [13]. Umbilical cord blood was collected from the umbilical veins after neonatal delivery, with informed consent from the pregnant mothers. MSCs were isolated by separating mononuclear cells (MNCs) using a Ficoll-Hypaque solution (d=1.077 g/cm3; Sigma, St. Louis, MO, USA). MNCs were transferred into a-minimum essential medium (a-MEM; Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with fetal bovine serum (FBS; Gibco, Life Technologies, Carlsbad, CA, USA). They were then seeded in culture flasks at a density of 5×105 cells/cm2. Cells were maintained in a humidified 5% CO2 chamber at 37℃ and colonies composed of spindle-shaped cells were formed. Once cells were 50% confluent, they were harvested after treatment with 0.25% (w/v) trypsin-EDTA (Gibco) and were reseeded for expansion. Three different hUCB-MSCs were prepared and they differed based on only the original UCB donor.
The prepared cells were assigned to either a control group or 1 of the 3 experimental groups. Group 1 rats (control) were administered 1X PBS intraparenchymally 48 h after MCAO. Group 2 rats were injected intraparenchymally with 5 µl total volume of hUCB-MSCs (G19, 5.0×105 in 1XPBS) 48 h after MCAO. Group 3 rats were injected intraparenchymally with 5 µl total volume of hUCB-MSCs (G16, 5.0×105 in 1XPBS) 48 h after MCAO. Group 4 rats were injected intraparenchymally with 5 µl total volume of hUCB-MSCs (G5, 5.0×105 in 1XPBS) 48 h after MCAO.
Limb placement tests (LPTs) included 8 subtests and were performed 2 days after ischemia, as previously described [14]. Briefly, the test consisted of 3 domains: (1) visual forward, (2) visual lateral, and (3) proprioception. Visual forward was defined as the observation of forelimb flexion when holding the animal by the tail. The stretch of the forelimbs towards the table was evaluated as either a normal stretch (0 points) or abnormal flexion (1 point). Visual lateral was defined as the observation of a forelimb stretch after a stimulus was applied to the rat's whiskers when it was held by its trunk. The visual lateral was scored as follows: normal lifting (0 points) or abnormal lifting (1, 2, or 3 points, according to the times of normal stretch). Proprioception was defined as the observation of stepping up of the forelimb and hindlimb on the table after pulling down the forelimb and hindlimb below the level of the table. This occurred 3 times and was scored as normal lifting (0 points) or abnormal lifting (1, 2, or 3 points, according to the times of normal stretch). Therefore, when all 3 domains were assessed, the highest possible score was 10.
The rotarod test (Panlab, Barcelona, spain) consisted of an accelerating rod, which provided an index of forelimb and hindlimb motor coordination and balance [15]. The rat was placed on an accelerating rod, whose velocity was slowly increased from 4 rpm to 40 rpm within a 10 min period. The duration for which the rat remained on the accelerating, rotating rod was measured in seconds. A trial was terminated when the rat fell off the rotarod. The animals were trained with 3 trials per day for 3 days before MCAO to obtain stable baseline values. The rotarod data are presented as a percentage [(mean duration of the 3 post-MCAO trials/ duration of pre-MCAO baseline trials) ×100].
After anesthesia, the animals were intracardially perfused with saline, followed by ice-cold 4% paraformaldehyde (PFA; in PBS) for 10 min. Their brains were removed, post-fixed for 1 day at 4℃, and then transferred sequentially at one-day intervals into 10%, 20% and 30% sucrose until they sank to the bottom of the container. The tissues were embedded in OCT compound (Sakura Finetek, Inc., Torrance, CA), and frozen at -70℃ using dry ice. The brain sections were cut to a thickness of 30 µm with a cryostat (Leica CM 3000, Leica, Solms, Germany).
IMMUNOHISTOCHEMISTRY
The brain sections were washed with 1X PBS, permeabilized with PBS containing 0.1% saponin and 4% normal goat serum (NGS) for 30 min at 25℃, and blocked with PBS containing 0.05% saponin and 5% NGS for 30 min. Brain sections were permeabilized for 30 min with 0.1% Triton X, and incubated overnight at 4℃ with the following antibodies: mouse anti-nestin (1:400; Chemicon, Temecula, CA), mouse anti-GFAP (1:600; Chemicon, Temecula, CA), and rabbit anti-laminin (1:30; Sigma Aldrich, Inc., St. Louis, MO). Brain sections were incubated for 1 h at room temperature with fluorescence-labeled secondary antibodies raised against the respective hosts of the primary antibodies at a dilution of 1:500. The secondary antibodies (Molecular Probes, Invitrogen Co., Carlsbad, CA) included Alexa 488 and Alexa 594 goat anti-mouse IgG and goat anti-rabbit IgG.
The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was used to measure apoptotic cell death in the control and experimental groups. Using an
Fluorescent immunolabeled sections were imaged with a Meta confocal microscope (LSM model 510; Carl Zeiss MicroImaging, Inc., Jena, Germany) equipped with 4 lasers (Diode 405, Argon 488, HeNe 543 and HeNe 633). Each channel was separately scanned using a multitrack-photomultiplier tube (PMT) configuration to avoid bleeding between the fluorescent labels in order to visualize the labeled structures in relation to other cells.
The infarct size was measured with Image J (NIH, v1.38). The sections were scanned with a high-resolution scanner (Epson Perfection V700 photo), and the 2 hemispheres were compared using ImageJ. The infarct lesion was drawn using a graphic tablet tool and the size was summed and presented as a percentage of the size of the intact hemisphere. Infarct Size = lesion size / non-lesion size ×100.
Statistical analysis was performed using Graph-Pad Prism version 3.03 (GraphPad Software. Inc., San Diego, CA). All data are presented as mean±SEM. A repeated-measures analysis of variance (ANOVA) was performed on all behavioral data. The quantification of the immunofluorescent staining in the control and experimental groups was performed using the Student's t-test.
RESULTS
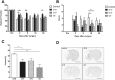
Abnormal motor performance caused by motor cortex infarcts was assessed by the modified LPT and rotarod. Each experimental group was administered a different lot number of hUCB-MSCs (e.g., G5, G16, G19) and it was compared to the control group. There were no significant differences in the neurological outcomes before cell transplantation. However, there was significant functional improvement in both motor tests in the G19 and G5 hUCB-MSC-treated groups starting 7 days after cell injection (therefore, 9 days after MCAO surgery) compared to the control group (rotarod and LPT: *p<0.05; Fig. 1A). There was no improvement in the G16 hUCB-MSC-treated group compared to the G19 and G5 hUCB-MSC-treated groups in either the rotarod (Fig. 1A) or the LPT (Fig. 1B). Therefore, motor dysfunction can be improved with hUCB-MSC administration without regard to the donor type.
To determine whether hUCB-MSCs could reduce the infarct size in the ischemic brain, brain tissue was stained with cresyl violet (Nissl staining). Infarcted lesions were mainly concentrated in the cortex and striatum at 28 days after hUCB-MSC injection. The entire ischemic tissue area was transformed into cysts. The relative infarct size in all hUCB-MSC- treated experimental groups was significantly smaller than that in the control group (*p<0.05; Fig. 1C and D). Therefore, hUCB-MSC administration can reduce stroke-induced infarct size.
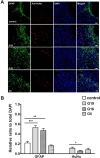
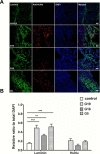
To determine whether donor-dependent hUCB-MSC administration enhanced angiogenesis in endothelial cells or endogenous astroglia, we performed double-labeled immunohistochemistry with GFAP and anti-human nuclei, and laminin and anti-human nuclei. hUCB-MSC treatment increased the percentage of GFAP- and laminin-positive cells in the ischemic boundary zone at 28 days after MCAO ischemic injury compared to the control group (Fig. 2A, B and 3A, B). There was no additional difference in the percentage of GFAP- and laminin-positive cells between the G19, G16, and G5 hUCB-MSC-treated groups. Co-localization of anti-human nuclei and GFAP or laminin was confirmed through double labeled immunofluorescence. GFAP expression was limited to a cell population in the border zone of the ischemic cortex. Expression of anti-human nuclei was located near GFAP and laminin positive populations of cells.
It was possible to determine approximately the mount through a measure of Human Nuclei (HuNu) positive cells. On average, on the basis of the measured mean value in the 1 mm×1 mm square, the number of surviving cells in each group was 88.8×103±1023 (G19), 33.6×103±2012 (G16), and 55.2×103±1286 (G5). The equivalent value based on the amount of administered cells was 17.76% (G19), 6.72% (G16), and 11.04% (G5).
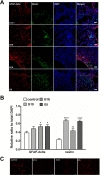
To determine whether hUCB-MSC administration induced neurogenesis in a donor-dependent manner, we performed immunofluorescent staining of nestin and GFAP-delta (Fig. 4A). Nestin and GFAP-delta expression was primarily located throughout the brain in the ischemic boundary zone. The sections were analyzed with Image Pro Plus software. The number of cells positive for nestin and GFAP-delta, markers of neurogenesis, was significantly increased from the ipsilateral striatum to the ischemic striatum in all hUCB-MSC-treated groups compared to control group (Fig. 4B). There were no differences between the G19, G16, and G5 hUCB-MSC-treated groups. Furthermore, hUCB-MSCs significantly reduced the density of apoptotic cells in the ischemic brain regardless of the donor, as indicated by the reduced number of TUNEL-positive, DNA-fragmented cells (Fig. 4C).
DISCUSSION
It is now well established that hUCB-MSCs possess core stem cell properties, such as self-renewal, lineage-specific differentiation and tissue engraftment, which are largely influenced by the local microenvironment and soluble molecular signals. hUCB-MSCs are a very promising cell therapy whose procedures have yet to be clearly delineated. However, because viability, the phenotype and the differentiation potential of hUCB-MSCs can change with each donor, it is important to understand whether hUCB-MSCs have different effects in ischemic modeling depending on donor cell origin.
Although cell donors may influence the therapeutic potential of hUCB-MSCs, our data indicated that these 3 types of hUCB-MSCs express a common mesenchymal stem lineage in the ischemic boundary zone. The infarct volume in the ischemic brain treated with 3 different types of hUCB-MSCs did not differ between the groups. However, the groups treated with 3 types of hUCB-MSCs had a lower infarct volume compared to the control group, which is consistent with the behavioral testing results (Fig. 1).
In order to further determine which neurochemical factors may have influenced our behavioral data, we stained brain tissue markers for neurogenesis and angiogenesis. hUCB-MSCs persisted in the ischemic boundary zone (Fig. 2) and survived up to 4 weeks after injection. Rats administered G19 hUCB-MSCs had a higher survival rate, which correlated with GFAP-expressing astroglial cells. Previously, studies have reported that adult neural stem cells resulted in astroglia-induced neurogenesis [16], which has a very important role in the repair process and inflammation [17]. Our data are consistent with these previously reported findings. hUCB-MSCs increased vessel formation in ischemic condition regardless of the donor (Fig. 3). The angiogenic reaction was increased at hUCB-MSC-administered sites and it correlated with the amount of hUCB-MSC survival in the ischemic boundary zone. hUCB-MSCs were used as a successful stem cell therapy tool for Buerger's disease and an ischemic limb disease animal model [18] and they promoted angiogenic effects in myocardial infarction [19]. Nestin and GFAP-delta, which are associated with neurogenesis, were increased in the ischemic boundary zone of rats in the 3 groups that received G19, G16, and G5 hUCB-MSCs (Fig. 4).Although the nestin and GFAP-delta expression patterns did not differ among the 3 hUCB-MSC-treated groups, we hypothesize that neurogenesis was increased through the hUCB-MSC treatment, which improved the MCAO-induced motor dysfunction. Many studies have reported that stem cells express nestin, a hallmark of adult neurogenesis [20,21,22], and that GFAP-delta is involved in adult neurogenesis [23].
Our results indicate that the hUCB-MSC profiles were similar, although not identical, to UCB-derived MSCs, reinforcing the idea that these 2 cell populations have the same cellular identity, and strong commonalities in their cellular characteristics and differentiation potential. hUCB-MSCs from diverse donors expressed very similar factors and reduced the behavioral deficits in the ischemic model. The cytokine secretion profile was highly conserved among BM-derived MSCs from different donors of diverse races, ages and sexes. The conserved secretion pattern suggests that the molecular profile of these cells is not greatly influenced by the individual donor characteristics [11,24] . However, there are some possible limitations in the current study that should be addressed. Our data regarding the characterization of hUCB-MSCs (G19, G16, and G5) is lacking. As an alternative, we reported angiogenic profiling of hUCB-MSCs in hypoxic condition [25], Although we did not conduct a specific cytokine profiling assay in hUCB-MSCs, our results revealed positive behavioral, neurogenic and angiogenic effects regardless of the individual hUCB-MSC donors. Further investigation is necessary to elucidate the cytokine and growth factor profiles of different donor types for future clinical trials using patient specific hUCB-MSCs.
In conclusion, locally injected hUCB-MSCs were capable of rescuing the detrimental effects of ischemic lesions in the striatum by increasing neurogenesis and angiogenesis, regardless of the original hUCB-MSC donor. hUCB-MSCs from different donors may show slightly different stem cell traits, but they are still capable of healing the diseased brain, regardless of the original donor.
Figures
References
- Alberts MJ. Diagnosis and treatment of ischemic stroke. Am J Med 1999;106:211-221.
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2008;2:313-319.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-147.
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005;33:1402-1416.
- Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294-1301.
- Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med 2005;139:504-509.
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009;5:54-63.
- Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res 2010;20:510-518.
- Martins JP, Santos JM, de Almeida JM, Filipe MA, de Almeida MV, Almeida SC, Água-Doce A, Varela A, Gilljam M, Stellan B, Pohl S, Dittmar K, Lindenmaier W, Alici E, Graça L, Cruz PE, Cruz HJ, Bárcia RN. Towards an advanced therapy medicinal product based on mesenchymal stromal cells isolated from the umbilical cord tissue: quality and safety data. Stem Cell Res Ther 2014;5:9.
- Hwang JH, Shim SS, Seok OS, Lee HY, Woo SK, Kim BH, Song HR, Lee JK, Park YK. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci 2009;24:547-554.
- Park CW, Kim KS, Bae S, Son HK, Myung PK, Hong HJ, Kim H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells 2009;2:59-68.
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84-91.
- Jin HJ, Nam HY, Bae YK, Kim SY, Im IR, Oh W, Yang YS, Choi SJ, Kim SW. GD2 expression is closely associated with neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells. Cell Mol Life Sci 2010;67:1845-1858.
- De Ryck M, Van Reempts J, Borgers M, Wauquier A, Janssen PA. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke 1989;20:1383-1390.
- Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma 1994;11:187-196.
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature 2002;417:39-44.
- Hauwel M, Furon E, Canova C, Griffiths M, Neal J, Gasque P. Innate (inherent) control of brain infection, brain inflammation and brain repair: the role of microglia, astrocytes, "protective" glial stem cells and stromal ependymal cells. Brain Res Brain Res Rev 2005;48:220-233.
- Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, Lee YS, Lee KS, Park HK, Kang KS. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buergers disease and ischemic limb disease animal model. Stem Cells 2006;24:1620-1626.
- Ma N, Stamm C, Kaminski A, Li W, Kleine HD, Müller-Hilke B, Zhang L, Ladilov Y, Egger D, Steinhoff G. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovasc Res 2005;66:45-54.
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, DiLeone RJ, Greer CA, Mandyam CD, Eisch AJ. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci 2007;27:12623-12629.
- Silkoff PE, Romero FA, Gupta N, Townley RG, Milgrom H. Exhaled nitric oxide in children with asthma receiving Xolair (omalizumab), a monoclonal anti-immunoglobulin E antibody. Pediatrics 2004;113:e308-e312.
- Divya MS, Roshin GE, Divya TS, Rasheed VA, Santhoshkumar TR, Elizabeth KE, James J, Pillai RM. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res Ther 2012;3:57.
- van Strien ME, van den Berge SA, Hol EM. Migrating neuroblasts in the adult human brain: a stream reduced to a trickle. Cell Res 2011;21:1523-1525.
- Jeong JA, Ko KM, Bae S, Jeon CJ, Koh GY, Kim H. Genome-wide differential gene expression profiling of human bone marrow stromal cells. Stem Cells 2007;25:994-1002.
- Park HW, Moon HE, Kim HS, Paek SL, Kim Y, Chang JW, Yang YS, Kim K, Oh W, Hwang JH, Kim JW, Kim DG, Paek SH. Human umbilical cord blood-derived mesenchymal stem cells improve functional recovery through thrombospondin1, pantraxin3, and vascular endothelial growth factor in the ischemic rat brain. J Neurosci Res 2015;93:1814-1825.



