Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2017; 26(1): 55-65
Published online February 28, 2017
https://doi.org/10.5607/en.2017.26.1.55
© The Korean Society for Brain and Neural Sciences
Effect of Single and Double Administration of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Following Focal Cerebral Ischemia in Rats
Hyung Woo Park1,2,3, Yona Kim1,2,3, Jong Wook Chang5, Yoon Sun Yang4, Wonil Oh4, Jae Min Lee1, Hye Ran Park6, Dong Gyu Kim1,2,3 and Sun Ha Paek1,2,3*
1Department of Neurosurgery, Seoul National University College of Medicine, Seoul 03080, 2Cancer Research Institute, Seoul National University College of Medicine, Seoul 03080, 3Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul 03080, 4Biomedical Research Institute, MEDIPOST Co., Ltd, Seoul 13494, 5Stem Cell & Regenerative Medicine Center, Research Institute for Future Medicine, Samsung Medical Center, Seoul 06351, 6Department of Neurosurgery, Soonchunhyang University Hospital, Seoul 31151, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-2072-3993, FAX: 82-2-744-8459
e-mail: paeksh@snu.ac.kr
Stem cell therapies are administered during the acute phase of stroke to preserve the penumbral tissues from ischemic injury. However, the effect of repeated cell therapy during the acute phase remains unclear. In this study, we investigated and compared the functional outcome of single (two days post-injury) and repeated (two and nine days post-injury) treatment with human umbilical cord derived mesenchymal stem cells (hUCB-MSCs) after middle cerebral artery occlusion (MCAO). The rotarod and limb placement tests were utilized to investigate functional outcomes, while infarct volume and tissue damage were measured by immunofluorescent staining for neovascularization, neurogenesis, apoptosis, and inflammation in the penumbral zones. We observed notable motor dysfunction and a significant decrease in infarcted brain volume, as well as increases in neurons and vessels in both single and repeated hUCB-MSC treatments compared to the control group. Interestingly, repeated administration of hUCB-MSCs was not found to elicit additional or synergistic improvements over monotherapy. This study suggests that a clearer understanding of the therapeutic window after stroke will facilitate the development of more efficient treatment protocols in the clinical application of stem cell therapy.
Keywords: Human umbilical cord blood mesenchymal stem cell, Ischemia, repeat therapy, Angiogenesis, Neurogenesis
Administration of human stem cells has been reported to ameliorate functional deficits after middle cerebral artery occlusion (MCAO) in rats [1,2,3,4,5,6,7]. However, there is an unmet need to develop effective treatments with a wide therapeutic window capable of restoring neural function and reducing disabilities associated with stroke. One novel therapeutic option is multiple administration of cell therapy following stroke. Previous research has shown that two intravenous treatments of autologous mesenchymal stem cell (MSCs) were effective in patients with ischemic stroke [8]. Furthermore, it has reported that repetitive administration of MSCs induced formation and growth of new nerve cells in a neonatal hypoxia model, and oligodendrocyte restoration of the corticospinal tract [9]. In a recent study, multiple administrations of stem cells was more effective than MSCs based immune modulation in treating stroke [10,11]. However, unlike previous reports on the effects of repetitive administration of stem cells on stroke, it remains unknown whether multiple injections of MSCs are superior to a single injection [12,13].
Previous reports suggest that two days after MCAO model formation is the most effective time point for human umbilical cord derived mesenchymal stem cell (hUCB-MSC) treatment [14]. Further, endogenous neurogenesis has been shown to peak seven days after experimental MCAO [15]. In light of this, we hypothesized that additional hUCB-MSC treatment seven days after MCAO would improve functional recovery after stroke.
Currently, there are no published trials regarding the beneficial effects of repeated administration of hUCB-MSCs in ischemic-stroke disease. However, repeated administration of hUCB-MSCs to patients with large acute myocardial infarction resulted in a significant increase in left ventricular ejection fraction and a decrease in myocardial infarct size compared with single intracoronary injection [16].
Therefore, we examined whether repeated hUCB-MSC administration in rats after ischemic insult would improve the outcome of MCAO model. Unexpectedly, however, we have found out that no significant therapeutic improvements following repeated treatments were observed although neurogenesis, angiogenesis, and anti-inflammation are found to be significantly activated in monotreated rats.
MATERIALS AND METHODS
Animals
Thirty adult male Sprague Dawley rats (200~250 g) were group housed in a temperature-controlled room with free access to food and water under 12 hour light-dark conditions. After surgery, twenty three of the thirty have survived and consequently, a total of twenty three rats were randomly assigned to post single two days (D2, n=7) or post double two and nine days (D2+7, n=5) or PBS-only (1X PBS single post two days, n=11) groups. All of the animals were cared and treated in accordance with the Guide for Care and Use of Laboratory Animals of the Clinical Research Institute of Seoul National University Hospital (IACUC No. 11-0114).
MCAO surgical procedure
Transient MCAO was induced with the intraluminal vascular occlusion method, as has been previously described [17]. The animals were anesthetized with xylazine (10 mg/kg, i.p.) and Zoletil 50 (75 mg/kg, i.p.). By using blunt dissection, the right common carotid, external carotid, internal carotid, and ptery-gopalatine arteries were exposed and isolated from the surrounding tissue. An embolus was inserted through the external carotid, into the internal carotid, and up to the origin of the MCA. Once in place, the embolus was tied in permanently and the incision closed. One and half hours after MCAO, reperfusion was performed by withdrawal of the suture. After recovery from anesthesia, animals were allowed free access to food and water.
Cell preparation and transplantation
This study was approved by the Institutional Review Board of MEDIPOST Co., Ltd (IRB No. 201002-01) and hUCB-MSCs were separated and maintained as described previously [18]. Umbilical cord blood was collected from umbilical veins after neonatal delivery with informed consent of the pregnant mothers. hUCB-MSCs were isolated by separation of mononuclear cells (MNCs) using a Ficoll-Hypaque solution (d=1.077 g/cm3; Sigma). After transferring into minimum essential medium (MEM; Gibco) supplemented with fetal bovine serum (FBS; Gibco), MNCs were seeded in a culture flask at 5×105 cells/cm2. Cells maintained in a humidified 5% CO2 at 37℃ formed colonies of spindle-shaped cells. At 50% confluence, cells were harvested after treatment with 0.25% (w/v) trypsin-EDTA (Gibco) and were reseeded for expansion. Prepared cells were administrated into three experimental groups at the anteroposterior, +1.0 mm from bregma, laterally +3.0 mm and dorsoventrally -5.0 mm, according to the stereotaxic atlas for Paxino and Watson (1986): In group one, rats were given 1X PBS injected intrastriatally two days after MCAO. In group two, rats were given hUCB-MSCs (5.0×105 cells) in 5 µl total volume (1X PBS) injected intrastriatally two days after MCAO. In group three, rats were given hUCB-MSCs (5.0×105 cells) in 5 µl total volume (1X PBS) injected intrastriatally two and nine days after MCAO.
Behavioral testing
Limb placement tests (LPTs)
LPTs included eight subtests and were performed two days after ischemia as previously described [19]. Briefly, the test consists of three domains: (1) visual forward, (2) visual lateral, and (3) proprioception. Visual forward measures forelimb flexion by holding up the tail and evaluating the stretch of the forelimbs towards the table: normal stretch, 0 points; abnormal flexion, 1 point. Visual lateral is a measure of forelimb stretch by stimulating the rats whiskers while the examiner holds the rats trunk. The visual lateral is scored by 0, 1, 2 and 3 points: normal lifting, 0 points; abnormal lifting, 1, 2, 3 points according to the times of normal stretch. Proprioception measures the upward movement of the forelimb and hindlimb on a table after pulling the forelimb and hindlimb down below the level of the table. This is repeated three times and scored as follows: normal lifting, 0 points; abnormal lifting, 1, 2, 3 points according to the times of normal stretch. Therefore, the highest total score is 10.
Rotarod test
An accelerating rotarod (Panlab) provides an index of fore and hindlimb motor coordination and balance [20]. The duration that the rat remained on the accelerating rotating rod was measured. The velocity was slowly increased from 4 rpm to 40 rpm within ten minutes. A trial was terminated when the rat fell from the rotarod. The animals were trained three trials per day for three days before MCAO to obtain stable baseline values (in seconds). Motor test data are presented as percentage of mean duration (three trials) on the rotarod compared with the internal baseline values.
Measurement of infarct size in rat brain slices
Measurement was conducted as previously described [17]. In briefly, we picked five slides between the start and endpoint of infarct. Cresyl violet staining of tissue sections was carried out in a conventional manner. Each stained section was scanned by high resolution scanner (Epson Perfection V700 photo), and each hemisphere was compared by Image J. The infarct lesion was drawn using a graphic tablet tool, and the relative infarct size was expressed as a percentage, which was calculated by comparing the lesioned hemisphere with the intact hemisphere. The size was presented as percentage of the corresponding intact tissue. 
Tissue preparation and immunohistochemistry
After completing behavioral testing, animals were perfused transcardially with 50 ml of 0.9% NaCl (with heparin), followed by 50 ml of ice-cold 4% paraformaldehyde. Tissue remained in ice-cold 4% paraformaldehyde for one day and was sequentially transferred at one-day intervals into 10%, 20% and 30% sucrose, respectively, until it sank to the bottom of the container. Tissue was then sectioned at 30 µm using a cryostat and sections were stored at -20℃ until immunohistochemistry was performed.
Transplanted hUCB-MSCs were identified with a human-specific monoclonal antibody to nuclear antigen (MAB 1281; 1:500; Chemicon, Temecula, CA). After blocking in normal goat serum, sections were double labeled with mouse anti-human nuclei and rabbit anti-laminin (1:400; Sigma), mouse anti-human nuclei and rabbit anti-GFAP (1:400; Millipore). In addition, single labeling of mouse anti-nestin (1:400; Millipore), mouse anti-GFAP-delta (1:500; Millipore), anti-rabbit COX2 (1:400; abcam), anti-rabbit TNFα (1:200; abcam) and anti-rabbit TGFβRII (1:500; Millipore) was performed overnight at 4℃. On the following day, sections were rinsed and incubated in secondary antibody conjugated to rhodamine, FITC (1:1000; Alexa 594 and 488; Molecular Probes). The sections were washed, mounted, and coverslipped with Vectashield mounting medium including DAPI (Vector Laboratories, Burlingame, CA) prior to examination under epifluorescence on an Olympus BX60 microscope. To verify that rhodamine labeling was specific to the hUCB-MSCs, the sections were also observed under theisothiocynate (FITC) wavelength. Any labeled cell observed under both conditions was determined to not be a hUCB-MSCs.
TUNEL assay
Apoptotic cells can be detected by terminal deoxynucleotidyl transferase(TdT)-mediated dUTP-biotin nick end labeling (TUNEL) assays in the control and experimental groups with the
Statistical analysis
Statistical analysis was performed using Graph-Pad Prism version 3.03 (GraphPad Software. Inc., San Diego, CA). All data are reported as mean±SEM. The quantified data for laminin, GFAP, human-nuclei, nestin, GFAP-delta, TUNEL, COX2, TNFα and TGFβRII in the control and experimental groups were analyzed by Student's t-test. The resulting projection image was loaded into image analysis software (Image-Pro Plus, Media Cybernetics Co., Silver Spring, MD). A similar threshold was set for all images, and the area of specific immunoreactivity was measured using an image analyzer. Immunoreactivity was then expressed as the average number of positive cells per cubic millimeter. For the behavioral studies, results were analyzed by two-way repeated-measures ANOVA and the infarct size were analyzed by ANVOVA.
Limited therapeutic effects of repeated hUCB-MSC treatment
Additional hUCB-MSC administration did not improve functional outcome
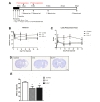
To evaluate the therapeutic effects of repeated treatment, rats were treated with 5.0×105 of hUCB-MSCs into the ischemic zone (the dorsolateral striatum and the cortex) two and nine days after inducing brain ischemia with MCAO. We previously have conducted the experiment regarding determining the appropriate number of cells in our previous published paper (
Infarct size does not change with repeated administration of hUBC-MSCs
The control group infarct volume was around 43% of the intact contralateral hemisphere. Infarct volume spontaneously decreased to 45-52% in control animals after 28 days, while the infarct volume of both single and repeated hUCB-MSC treated groups continued to decrease (Fig. 1D, 28.93±5.05%, 30.05±6.0%). However, the difference between the two treatment groups was not significant. These data indicate that the higher motor function observed in both hUCB-MSC injected groups correlated to less ischemic damage and higher tissue integrity in the host brain 28 days after MCAO; however, repeated dosing did not improve any endpoints.
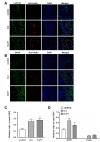
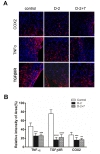
Single and repeated hUCB-MSC treatment similarly increased angiogenesis
To determine whether the second administration of hUCB-MSCs further enhanced angiogenesis of endothelial cells, we performed double immunohistochemical analysis. Single treatment of hUCB-MSCs at day two increased the percentage of laminin positive cells in the ischemic boundary zone at 28 days after MCAO compared to control animals. Laminin, a major family of extracellular matrix molecules present in basement membranes. it was reported that laminin-111, -411, -511 and -332 and their associated signalling that regulates cell behaviour and angiogenesis under normal and pathological conditions [21]. However, we observed no additional increase in the percentage of laminin positive cells after the second injection of hUCB-MSCs at day seven (Fig. 2A and C).
Ischemia induced expression of GFAP was not different between single and repeated administration of hUCB-MSCs
Colocalization of anti-human nuclei and GFAP was confirmed through double fluorescence labeling. Expression of GFAP was limited to a population of cells in the border zone of the ischemic cortex. Expression of anti-human nuclei was observed near the GFAP positive cells. While both hUCB-MSC treatment groups, D-2 and D-2+7, significantly increased the number of GFAP positive cells, no statistical difference was found between single and repeated administrations of hUCB-MSCs (Fig. 2B and D).
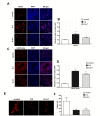
hUCB-MSC treatment increased the expression of nestin and GFAP-delta but repeated administration did not improve outcomes compared to single administration
To determine whether improved motor function after hUCB-MSC treatment was associated with increased neurogenesis, immunohistochemistry was performed with nestin and GFAP-delta in control, D-2 and D-2+7 groups. In the adult human, GFAPδ is a marker for neural stem cells. GFAPδ is highly expressed in the adult human SVZ. It co-labels with stem cells markers such as sex-determining region Y-box 2 (Sox2) and nestin, as well as the cell division markers minichromosome maintenance complex component 2 (MCM2) and proliferating nuclear antigen (PCNA) [22]. D-2 treatment animals exhibited significantly increased nestin and GFAP-delta positive cells in the ischemic boundary zone 28 days after MCAO. The number of nestin and GFAP-delta positive cells was not statistically different between D-2 and D-2+7 hUCB-MSC treatment groups (Fig. 3A~D, 37.8±1.4%, 52.8±5.5%, *p<0.05).
hUCB-MSC administration promotes anti-inflammatory effects
To confirm that hUCB-MSC treatment promotes anti-inflammation, we performed immunohistochemical labeling of inflammatory related proteins, including; tumor necrosis factor alpha (TNFα), transforming growth factor, beta receptor III (TGFβRII), and cyclooxygenase-2 (COX2). Immunostaining for TNF-α and TGFβRIII indicated that inflammation was decreased in both hUCB-MSC treated groups, D-2 and D-2+7 (Fig. 4A and B). However, there was no significant difference between single and repeated hUCB-MSC treatment groups. These results indicate that hUCB-MSC treatment increases angiogenesis and decreases inflammatory responses after ischemic injury and that these effects were not associated with repeated number of the treatment.
We demonstrated that both single and repeated hUCB-MSC treatments after MCAO markedly improved motor function 28 days after the insult. However, the repeated treatment group (D-2+7) did not exhibit enhanced functional outcome compared to the single treatment group (D-2). Furthermore, there was no difference in infarct size between D-2 and D-2+7 groups. Notably, the group with additional treatment was not associated with improvement in functional outcomes in behavioral tests compared to the group with single injection. These findings indicate that the beneficial effect of the first and second administration of hUCB-MSC treatment depends on microenvironmental changes after stroke. Therefore, it is speculated that no functional effect of additional injection of MSCs might have been due to the response of hUCB-MSCs to the brain milieu.
It has been suggested that MSCs treatment decreases brain damage by inhibiting injurious processes, by replacing lost tissue, and/or by enhancing endogenous repair processes [1,5,23,24,25,26,27]. We detected a few anti-human nuclei positive cells indicative of transplant origin at 35 days after MCAO. hUCB-MSC treatment two days after stroke resulted in enhanced endogenous cell proliferation and survival, and differentiation of recently divided cells. These findings indicate that hUCB-MSC treatment stimulates endogenous cell proliferation, survival, and differentiation. In addition, inhibition of injurious processes may also contribute to the beneficial effects of MSC treatment [1,24,25].
Data in the present study showed that delayed cell administration disappeared when the treatment period was extended to seven days, indicating that this particular hUCB-MSC therapy only protects against acute ischemic neuronal death. Other post-ischemic events have been linked with this delayed neuronal damage and are independent of the initial insult. We hypothesized that the differential effect of hUCB-MSC treatment at two or two and nine days was dependent on the response of hUCB-MSCs to the brain milieu. In addition, GFAP infiltration, and neurogenic and angiogenic factor expression are similar in single and repeated treatment groups. These results suggest that changes in ischemic environment are time dependent.
Most affected genes were distributed among 12 functional categories in ischemic conditions [28]. Immediate early genes, transcription factors, and heat shock proteins were up-regulated as early as 30 minutes after MCAO, followed by the up-regulation of inflammation, apoptosis, cytoskeletal, and metabolism genes, which peaked within 4~24 hours after injury (Table 1). Neurotrophic growth factors exhibited sustained up-regulation as early as 24 hours after MCAO and persisted through seven days post-injury. Three classes of genes were down-regulated with distinct temporal patterns: ion channel genes and neurotransmitter receptor genes were down-regulated for 8~24 hours after injury, whereas synaptic protein genes were down-regulated between 3~7 days after MCAO. Over 60% of the synaptic protein genes identified were up-regulated in the initial phase of brain ischemia. As a functional group, most synaptic protein genes showed distinct down-regulation during the delayed phase of ischemic injury. Synaptic proteins are involved in the regulatory process of synaptic-vesicle cycling at nerve terminals and play essential roles in neurotransmitter exocytosis by controlling docking, fusing, and trafficking of synaptic vesicles at the synapse upon cell stimulation.
Although many genes were expressed differentially at multiple points, it is interesting to note that there was limited overlap of molecular activities between early and late responsive genes. Although apoptosis and inflammation promote neuronal death, endogenous defense systems are also activated after ischemic attack to protect against cell death. In this study, an increasing number of growth factors were up-regulated throughout the seven days of injury period. It has been shown that growth factors enhance neuronal survival, promote neo-angiogenesis, and may also participate in phagocytic activity after global or focal brain ischemia [29,30]. These neural repair mechanisms require the participation of microglial cells and astrocytes, which are more viable at late stages of ischemia and therefore able to aid in tissue remodeling and long-term recovery. Activation of astrocytes and glial cells have also been purported to maintain cellular survival after brain ischemia, as evidenced by increased expression of some cytoskeletal genes such as GFAP, vimentin, and nestin throughout the seven days injury period.
The use of MSCs as a therapeutic modality for stroke is attractive. Preclinical studies have established the potential role of MSCs to be a useful and safe treatment for stroke in humans. After peripheral injection, MSCs cross the blood-brain barrier preferentially in areas that have experienced brain damage [31,32]. Intravenous application of MSCs reduced apoptosis and promoted endogenous cellular proliferation after stroke [1]. Animal models of stroke were also found to undergo functional improvementsfollowing MSC transplantation [5]. However, most of the experimental cell therapies used to improve motor function have been performed during the acute phase, and therefore, it is difficult to determine whether acute phase cell therapy is associated with he effect of spontaneous recovery. As a functional group, most synaptic protein genes showed distinct down-regulation at the delayed phase of ischemic injury. Synaptic proteins are involved in the regulatory process of synaptic-vesicle cycling at nerve terminals and play essential roles in neurotransmitter exocytosis by controlling docking, fusing, and trafficking of synaptic vesicles at the synapse upon cell stimulation. Compared to this previous report, initial positive factors that were secreted by MSCs decrease gradually towards the end, coinciding with our results that additional treatment at day nine may not elicit any synergistic effects.
Although our study provides meaningful results associated with therapeutic consequences of MSCs in MCAO model, it has several limitations.
First, additional control group in group 3 should have received PBS 2 and 9 days after MCAO model was generated to compare the therapeutic efficacy between repeated and single administration. Second, although additional cells were transplanted in D-2+7 group as opposed to D-2 group, the number of HuNu cells appears to be the same in these 2 groups. A possible explanation for such phenomenon is not yet clear. And last, although intrastriatal injection was demonstrated to be an effective method of cell transplantation in our research, such an injection method has been known to be invasive compared to intra-arterial or intravenous injection methods, and therefore, clinical application of intrastriatal injection seems to be implausible at this point.
| Gene name | GenBank accession | Early 30 min | Peak within 24 h | Sustained 7 days | Reference | |
|---|---|---|---|---|---|---|
| Up regulation gene | c-fos | X06769 | Y | N | N | Neumer, 2000; Sharp et al., 2000 |
| c-jun | X17163 | Y | N | N | ||
| Heat shock protein 27 | M86389 | Y | N | N | Jaattela, 1999; Neumer, 2000 | |
| Heat shock protein 60 | U68562 | Y | N | N | ||
| Heat shock protein 70 | Z27118 | Y | N | N | ||
| Caspase-2 | AP025671 | N | Y | N | MacManus and Linnik, 1997 | |
| Bax | U49729 | N | Y | N | ||
| IL-1β | U14647 | N | Y | N | ||
| Bcl-x | U72349 | N | Y | N | ||
| Bcl-2 | S72122 | N | Y | N | ||
| IL-6 | M26744 | N | Y | N | Abraham et al., 2002 | |
| TNF-α | L00981 | N | Y | N | ||
| IESR-JE(MCP-1) | X17053 | N | Y | N | ||
| MIP-1α | U22414 | N | Y | N | ||
| ICAM-1 | D00913 | N | Y | N | ||
| ELAM-1 | L25527 | N | Y | N | ||
| NF-κB | L26267 | N | Y | N | ||
| GFAP | AP028784 | N | N | Y | Cuevas, 1997; Wu and Pardridge, 1999 | |
| Vimentin | X62952 | N | N | Y | ||
| Nestin | M34384 | N | N | Y | ||
| Down regulation gene | K+ channel | Z34264 | N | N | Y | Neumer, 2000; Yao et al., 2003; Williams and Tortella, 2002; Williams et al., 2003 |
| Na+ channel I | M22253 | N | Y | N | ||
| Chloride channel-2 | AF005720 | N | Y | N | ||
| Acid gated ion channel | AJ006519 | N | Y | N | ||
| Ca2+ channel | M89924 | N | Y | N | ||
| mGluR 5 | D10891 | N | Y | N | Raghavendra Rao et al., 2002 | |
| NMDA receptor 2A | D13211 | N | Y | N | Hsu et al., 1998; Friedman et al., 1 | |
| GABA receptor β1 | X15466 | N | Y | N | Francis et al., 1999 | |
| muscarinic acetylcholine receptor | M16409 | N | Y | N | Hsu et al., 1996 |
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res 2003;73:778-786.
- Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol 2006;199:56-66.
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 2006;84:1495-1504.
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res 2004;1007:1-9.
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 2002;59:514-523.
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 2005;136:161-169.
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci 2001;189:49-57.
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010;28:1099-1106.
- van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci 2010;30:9603-9611.
- Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells 2015;8:54-68.
- Vaes B, Van't Hof W, Deans R, Pinxteren J. Application of MultiStem(®) allogeneic cells for immunomodulatory therapy: clinical progress and pre-clinical challenges in prophylaxis for graft versus host disease. Front Immunol 2012;3:345.
- Omori Y, Honmou O, Harada K, Suzuki J, Houkin K, Kocsis JD. Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats. Brain Res 2008;1236:30-38.
- Shehadah A, Chen J, Kramer B, Zacharek A, Cui Y, Roberts C, Lu M, Chopp M. Efficacy of single and multiple injections of human umbilical tissue-derived cells following experimental stroke in rats. PLoS One 2013;8:e54083.
- Newcomb JD, Ajmo CT, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant 2006;15:213-223.
- Shin HY, Kim JH, Phi JH, Park CK, Kim JE, Kim JH, Paek SH, Wang KC, Kim DG. Endogenous neurogenesis and neovascularization in the neocortex of the rat after focal cerebral ischemia. J Neurosci Res 2008;86:356-367.
- Henning RJ, Burgos JD, Vasko M, Alvarado F, Sanberg CD, Sanberg PR, Morgan MB. Human cord blood cells and myocardial infarction: effect of dose and route of administration on infarct size. Cell Transplant 2007;16:907-917.
- Park HW, Moon HE, Kim HS, Paek SL, Kim Y, Chang JW, Yang YS, Kim K, Oh W, Hwang JH, Kim JW, Kim DG, Paek SH. Human umbilical cord blood-derived mesenchymal stem cells improve functional recovery through thrombospondin1, pantraxin3, and vascular endothelial growth factor in the ischemic rat brain. J Neurosci Res 2015;93:1814-1825.
- Jin HJ, Nam HY, Bae YK, Kim SY, Im IR, Oh W, Yang YS, Choi SJ, Kim SW. GD2 expression is closely associated with neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells. Cell Mol Life Sci 2010;67:1845-1858.
- De Ryck M, Van Reempts J, Borgers M, Wauquier A, Janssen PA. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke 1989;20:1383-1390.
- Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma 1994;11:187-196.
- Simon-Assmann P, Orend G, Mammadova-Bach E, Spenlé C, Lefebvre O. Role of laminins in physiological and pathological angiogenesis. Int J Dev Biol 2011;55:455-465.
- Mamber C, Kamphuis W, Haring NL, Peprah N, Middeldorp J, Hol EM. GFAP expression in glia of the developmental and adolescent mouse brain. PLoS One 2012;7:e52659.
- Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 2006;24:1054-1064.
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 2005;49:407-417.
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A 2008;105:14638-14643.
- van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Regeneration of the ischemic brain by engineered stem cells: fuelling endogenous repair processes. Brain Res Brain Res Rev 2009;61:1-13.
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol 2009;8:491-500.
- Lu XC, Williams AJ, Yao C, Berti R, Hartings JA, Whipple R, Vahey MT, Polavarapu RG, Woller KL, Tortella FC, Dave JR. Microarray analysis of acute and delayed gene expression profile in rats after focal ischemic brain injury and reperfusion. J Neurosci Res 2004;77:843-857.
- Cuevas P. Therapeutic prospects for fibroblast growth factor treatment of brain ischemia. Neurol Res 1997;19:355-356.
- Wu D, Pardridge WM. Neuroprotection with noninvasive neurotrophin delivery to the brain. Proc Natl Acad Sci U S A 1999;96:254-259.
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001;32:1005-1011.
- Eglitis MA, Dawson D, Park KW, Mouradian MM. Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport 1999;10:1289-1292.



