Articles
Article Tools
Stats or Metrics
Article
Review Article
Exp Neurobiol 2017; 26(4): 186-194
Published online August 31, 2017
https://doi.org/10.5607/en.2017.26.4.186
© The Korean Society for Brain and Neural Sciences
Toll-like Receptor 2: A Novel Therapeutic Target for Ischemic White Matter Injury and Oligodendrocyte Death
Jun Young Choi1,2 and Byung Gon Kim1,2*
1Department of Neurology, Ajou University School of Medicine, Suwon 16499, 2Department of Brain science, Ajou University School of Medicine, Suwon 16499, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-32-219-4495, FAX: 82-31-219-4530
e-mail: kimbg@ajou.ac.kr
Abstract
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
Despite paramount clinical significance of white matter stroke, there is a paucity of researches on the pathomechanism of ischemic white matter damage and accompanying oligodendrocyte (OL) death. Therefore, a large gap exists between clinical needs and laboratory researches in this disease entity. Recent works have started to elucidate cellular and molecular basis of white matter injury under ischemic stress. In this paper, we briefly introduce white matter stroke from a clinical point of view and review pathophysiology of ischemic white matter injury characterized by OL death and demyelination. We present a series of evidence that Toll-like receptor 2 (TLR2), one of the membranous pattern recognition receptors, plays a cell-autonomous protective role in ischemic OL death and ensuing demyelination. Moreover, we also discuss our recent findings that its endogenous ligand, high-mobility group box 1 (HMGB1), is released from dying OLs and exerts autocrine trophic effects on OLs and myelin sheath under ischemic condition. We propose that modulation of TLR2 and its endogenous ligand HMGB1 can be a novel therapeutic target for ischemic white matter disease.
Keywords: Oligodendrocyte, White matter, Ischemia, Toll-like receptor 2, High-mobility group box 1
INTRODUCTION
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
White matter is composed of axons, myelin sheaths and glial cells. A principal physiological role of white matter is the rapid electrical signal transduction, so called saltatory conduction. To achieve saltatory conduction, axons should be enwrapped with myelin sheath. Oligodendrocyte (OL) is responsible for making myelin sheaths that are critical for the saltatory conduction. Therefore, maintaining integrity of OLs and myelin sheaths in the white matter is a fundamental foundation for a variety of adequate brain functions [1].
Human is the only species having the brain in which the volume of white matter is larger than that of gray matter [2]. Because of the larger white matter in the brain, many neurological conditions were caused or accompanied by white matter damage. Among neurological diseases or disorders affecting the white matter, ischemic insult is one of the most commonly encountered disease entities in clinical practice. Despite the high prevalence and clinical significance of ischemic white matter disease, relatively less effort has been invested on researches focusing on the pathomechanism or finding therapeutic targets in ischemic white matter disease than in ischemic neuronal/gray matter injury [3].
Ischemic insults can provoke sterile inflammation in damaged tissue, so called sterile inflammation [4]. Sterile inflammation after ischemic stroke affects tissue damage, neuronal cell survival and finally neurobehavioral outcome. Toll-like receptor (TLR) family is one of the main executors for post-ischemic inflammation after interaction with danger associated molecular pattern protein (DAMP) like high mobility group box-1 (HMGB1) or heat shock protein family from damaged cells [5]. More than several studies have focused on TLRs in ischemic stroke to reduce tissue damage or enhance tissue recovery via modulation of neuroinflammation [6,7]. However, it has been increasingly recognized that TLRs play a lot more diverse role than regulation of neuroinflammation [8]. In line with these non-immune functions of TLRs in CNS, we have recently discovered a novel role of TLR2 and its endogenous ligand HMGB1 in protecting ischemic OL death and demyelination in white matter stroke model [9,10]
In this paper, we briefly review about clinical spectrum of ischemic white matter diseases and introduce recent updates on the pathophysiology of white matter stroke. We will also propose novel pathomechanisms underlying ischemic oligodendrocyte death/white matter injury and potential therapeutic targets particularly focusing on the HMGB1-Toll-like receptor 2 (TLR2) pathways based on our recent reports.
CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
Clinically, ischemic lesions involving white matter can be classified into three types. One is acute focal ischemic white matter lesion, so called the lacunar infarction [11], which is caused by occlusion of deep perforating arteries. The second entity is the leukoaraiosis [12], asymptomatic white matter lesion presumably caused by chronic ischemia, that can be detected only by neuro-imaging modalities such as the computerized tomography and the magnetic resonance imaging (MRI). Third type is subcortical ischemic vascular dementia (SIVD) caused by chronic diffuse ischemic condition in white matter [13].
Approximately 20% of all ischemic strokes consist of purely focal subcortical white matter lesions [14]. When major cerebral arteries are occluded, white matter is affected to an extent similar to that of the gray matter[15]. White matter involvement is associated with poor prognosis and chronic neurological deficit after ischemic stroke [16]. It is widely accepted that one of the reasons for the failure of many neuroprotective reagents in clinical trials is due to accompanying white matter lesions that cannot be benefitted from the drugs targeting only neuronal pathologies [17].
The term of leukoaraiosis was introduced in 1986 to describe ‘rarefaction of white matter’ [18]. Because the majority of white matter in human cerebrum is supplied by single source of long arteriole or artery, slight decrease of cerebral perfusion or brief period asymptomatic ischemia can cause insult to the white matter [19,20]. After the discovery that the leukoaraiosis was the result of hypoperfusion to the white matter, leukoaraiosis has been considered as incomplete infarction in the white matter and then recognized as one of the manifestations deriving from the cerebral small vessel diseases [21,22].
The Leukoariosis and Disability (LADIS) study has shown that the baseline leukoaraiosis severity affects general performance, depressive symptoms, urinary problems and cognitive performance even in grossly non-disabled elderly population. LADIS study also noted that the leukoaraiosis is not a static disease but may gradually worsen and contribute to the progression of cognitive dysfunctions [23,24,25,26]. While less than 5% of elderly population have symptomatic focal acute ischemic stroke, the leukoaraiosis is found in almost all of the population aged equal to or more than 85 years old [27,28]. The high prevalence of leukoaraiosis further highlights its clinical significance.
A classical definition of VaD is the cognitive impairments that adversely affect an individual's daily life caused by or associated with vascular factors [29]. According to several epidemiologic studies, VaD is the second most common form of dementia and more frequently found in Asians (about 30% of prevalence) [30,31,32] than in western countries (about 5~10%) [33]. There are several subtypes of VaD as follows: 1) multi-infarction dementia, 2) strategic infarction dementia, 3) subcortical ischemic vascular dementia (SIVD) and 4) mixed dementia (VaD+Alzheimer's disease) [34]. Among the above VaD subtypes, SIVD is a relatively homogenous entity, and pure SIVD without any confounding factors is more frequently found than previously thought [35]. Pathomechanism of the SIVD is probably overlapped with that of focal acute ischemic white matter stroke or leukoaraiosis, because combinations of repeated complete infarction in the white matter and/or incomplete infarction caused by arterial stenosis or occlusion are the main pathophysiology of SIVD [36].
The above three clinical entities highlight importance of therapeutic strategies targeting white matter. Currently, treatments of the white matter strokes encompassing the lacunar infarction, leukoaraiosis and SIVD are not different from those targeting strokes involving the gray matter. For example, general treatment strategies of ischemic white matter injuries are as follows: 1) to prevent further arterial occlusion or stenosis using antiplatelet agent with treatment of vascular risk factors such as hypertension, diabetes and hypercholesterolemia [37], 2) life style modifications such as stop smoking [38] and 3) cholinesterase inhibiting agent as symptomatic treatment in VaD patients [39]. The fact that the current therapeutic tools in clinic are no longer different from those for the strokes caused by steno-occlusion of larger cerebra arteries indicates that no specific reagent has been developed selectively for ischemic white matter injuries. More researches to elucidate cellular and molecular mechanisms of ischemic white matter stroke would be required for clinical development of a therapeutic reagent specifically targeting ischemic white matter injury.
ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
One of the biggest huddles to study pathophysiology of ischemic white matter is a lack of animal models that can adequately replicate pathology of human white matter stroke. Recently, many researchers have attempted to establish various animal models for ischemic white matter damage to reduce the gap between laboratory and clinical practice [3,40,41]. Animal models for ischemic white matter damage can be divided into models for focal ischemic white matter damage like lacunar infarction within the white matter and chronic diffuse ischemic white matter disease mimicking leukoaraiosis and/or SIVD [40,41]. To make a focal ischemic white matter lesion, stereotaxic injection of a vasoconstriction reagent has been used. In rats, endothelin-1 (ET-1), a potent vasoconstrictor, has been successfully used for this purpose [2,42]. Advantage of using ET-1 injection is to make a convincing focal ischemic lesion in any wanted area including white matter. In comparison, controversial reports exist on a focal ischemic mice model using ET-1. Horie et al. reported that ET-1 injection alone could not make convincing focal ischemic lesions in the white matter [43]. However, our lab and the others reported generation of reproducible lesions in the internal capsule or cingulum in mice using ET-1[9,44,45]. Despite its advantages, the model ET-1 has some drawbacks. OLs express ET-1 receptor and ET-1 is known to exert biological effects on OL migration and differentiation [46]. Moreover, astrocyte-derived ET-1 could inhibit remyelination in focal chemical demyelinating lesion [47]. Therefore, an alternative vasoconstrictor, eNOS inhibitor N5-(1-iminoethyl)-L-ornithine, dihydrochloride (L-NIO), has been recently introduced to make focal ischemic white matter damage [48].
Chronic diffuse ischemic white matter model is preferred to the above focal model since it allows researchers to address cognitive impairment that is encountered in patients with VaD, especially SIVD. The most popular and classical animal model for vascular dementia and leukoaraiosis is bilateral common carotid artery occlusion (BCCAO) in rat [49]. Since BCCAO is frequently lethal in mice, bilateral common carotid artery stenosis (BCCAS) using micro-coil was introduced in mice. In this model, glial activation and working memory deficits that occur in human SIVD were successfully replicated [50,51]. Because BCCAO and BCCAS models are not accompanied by obvious hippocampal damage, these models are suitable and appropriate to study SIVD [40]. However, these animal models possess a serious weakness that cannot be overlooked. The BCCAO and BCCAS models exhibiting diffuse ischemia were produced by sudden occlusion or stenosis of blood vessels, unlike the leukoaraiosis or vascular dementia encountered in human patients in which vascular occlusion slowly and progressively occurs. To overcome this limitation of BCCAO and BCCAS, a recent study developed a new white matter ischemia model in which occlusion of carotid arteries was gradually induced by application of an ameroid constrictor [52,53].
RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
Demyelination and OL degeneration constitute prominent main pathological findings in the brain with white matter lesions on MRI [54]. The Binswanger's dementia, the most severe form of SIVD, is also accompanied by marked demyelination and OL loss [55,56]. Since degeneration of myelin sheath results in a decrease conduction velocity, demyelination and OL should be primarily responsible for a range of neurological dysfunctions in the white matter stroke. Although it has been known for a long time that OLs are particularly vulnerable to ischemic stress [20], there is very limited information on cellular and molecular mechanisms that regulate OL death and resultant demyelination under ischemic stress. Several recent studies have started to shed novel insights into the pathophysiology and to provide interesting ideas on identifying therapeutic targets in ischemic white matter stroke.
In diffuse ischemic BCAS model in mice, oligodendrocyte progenitor cell (OPC) produces matrix-metalloproteinase 9 that is expressed in response to an ischemic insult and disrupts the blood brain barrier to facilitate ischemic injury [57]. In contrast to the lesion-aggravating effects of OPC, astrocytes in the same BCAS model release BDNF and the released BDNF can enhance OPC differentiation and proliferation of OL lineage cells to restore ischemic white matter damage [58]. These studies indicate that multiple cell types are involved and interact with each other and elucidation of cell-to-cell interaction is important to delineate upstream causative pathological events to identify meaningful therapeutic targets.
Another recent study showed that oxidative stress interferes with endogenous white matter repair by disrupting OPC differentiation and that free radical scavenger can be utilized as a potential therapeutic approach for white matter injury in VaD and stroke [59]. OPC differentiation to mature OL is blocked also by myelinassociated molecules. It was recently discovered that Nogo receptor blockade overcomes remyelination failure after white matter stroke, and more importantly, blocking Nogo receptor leads to significant post-stoke motor recovery in aged mice [60]. In addition, various therapeutic approaches have been employed in ET-1 induced focal white matter stroke model. Exogenous application of adipose-derived mesenchymal stem cells [61], BDNF [62], and extracellular vesicles [63] promoted white matter repair in this focal stroke model. These studies suggest that therapeutic approaches targeting restoration of white matter integrity could be a hopeful approach for the treatment of white matter stroke.
TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
Our lab has also introduced a new player in the pathophysiology of white matter stroke. Inflammatory responses after ischemic insults have been considered one of the main factors to aggravate tissue damage. TLRs are known as pattern recognition receptors that are critically implicated in the regulation of innate immunity. Extracellular domain of TLRs binds to either exogenous pathogens called pathogen-associated molecular pattern proteins (PAMP) mainly derived by bacterial wall component or endogenous danger-associated molecular pattern proteins (DAMP) originating from degraded extracellular matrix or intracellular contents released after cellular demise. After binding with PAMP or DAMP, TLRs activate its downstream signaling pathways and promote production of inflammatory cytokines in inflammatory cells [64]. In contrast to this traditional proinflammatory influence of TLR activation, recent studies demonstrated that TLR activation could also drive cellular survival or protective mechanisms against various cellular insults in non-inflammatory cells [65,66].
While neurons, astrocytes and microglia express a broad range of TLRs, OLs express only TLR2 and TLR3 [67,68,69]. What are the consequences of TLR activation in OLs? Stimulation of TLR3 with poly (I:C) on cultured OLs resulted in pronounced cell death [67]. In contrast, treatment of OLs with TLR2 agonists such as zymosan or Pam3CSK4 resulted in increased OL maturation and survival [67]. A recent study showed that TLR2 activation with hyaluronan, one of the extracellular matrices increasing after inflammatory demyelination like multiple sclerosis, delays OL maturation and inhibits remyelination process [70]. These studies collectively led us to speculate on potential influence of TLR2 on OLs and myelinated axon in white matter stroke model.
We employed ET-1 induced focal white matter stroke model [9]. In this model, profound loss of OL lineage cells and disappearance of immunoreactivity to myelin basic protein (MBP) were observed within the focal demyelinating lesion. Interestingly, TLR2 (-/-) mice showed larger ET-1 induced ischemic demyelinating area than that in wild type (WT) mice. Interestingly, the difference in lesion size was not due to altered post-ischemic inflammation because the extent of microglial cell infiltration and proinflammatory gene activation was comparable between WT and TLR2 (-/-) animals. The expansion of demyelinating lesion in TLR2 (-/-) mice was accompanied by twice as many apoptotic OLs as in WT. To demonstrate OL-intrinsic influence of TLR2 activation, primary cultured OLs from WT or TLR2 (-/-) mice were exposed to oxygen-glucose deprivation (OGD), an
HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
After establishing the cell-autonomous protective role of TLR2 in OGD-induced OL death, we sought to identify an endogenous ligand that elicits activation of TLR2 expressed in OLs [10]. Since the TLR2 activation occurred following OGD insult to dissociated OLs in culture, we reasoned that TLR2 activating ligand should be released from OLs rather than other cells or extracellular matrix. We screened presence of potential DAMPs known to be released from intracellular compartment in culture medium following OGD and found out a time-dependent accumulation of HMGB1 [10], which is a well-known TLR2 ligand. Extranuclear translocation of HMGB1 and its extracellular release from OLs under ischemic stress could be observed. The conditioned medium (CM) obtained from cultured OLs exposed to OGD protected separate cultured OLs from OGD-induced ischemic stress. However, the OL-OGD CM with HMGB1 immunodepleted did not exhibit the protective effects. In addition, exogenous application of HMGB1 could rescue OGD-induced OL death in a TLR2-dependent manner, and HMGB1 inhibition with glycyrrhizin aggravated OGDinduced OL death. The HMGB1-TLR2 axis activation was correlated with IκB-α degradation, ERK1/2 phosphorylation and CREB phosphorylation
The
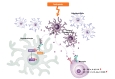
Taken together, we can speculate autocrine, cell-autonomous protective model of HMGB1-TLR2 axis in ischemic OL death (Fig. 1). Under ischemic condition, HMGB1 are released from nuclei of dying OLs. Released HMGB1 interacts with TLR2 on neighboring OLs in an autocrine manner. After interaction between TLR2 and released HMGB1, OLs turn on their pro-survival signaling pathway like ERK1/2 and CREB phosphorylation. Released HMGB1 also interacts with TLR2 on microglia but does not tip the balance between pro- and anti-inflammatory states because it increases the expression of both M1 and M2 markers in microglial cells. This model suggests a possibility to utilize an innate protective or recovery mechanism in ischemic OL death and illustrates an importance of understanding complex cell-to-cell interactions in the white matter stroke.
CONCLUSIONS AND FUTURE PERSPECTIVES
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
A still large gap lies between clinical practice and laboratory researches in ischemic white matter stroke, and therefore, huge unmet clinical needs exist. A series of our recent works and others have shed new insights on the pathomechanism of ischemic OL death and white matter injury. We proposed TLR2 as a novel therapeutic target to ischemic OL death and white matter stroke. However, there are still several remaining questions on TLR2 in the contexts of ischemic OL death and white matter injury. First, ischemic OL death was not completely rescued by HMGB1 alone in our recent report. This result suggests that there are other ligands on TLR2 to promote prosurvival effect or the presence of other receptors rather than TLR2. Second, the detailed intracellular prosurvival signaling pathway through TLR2 in OL has not yet been elucidated. Discovering more detailed intracellular prosurvival signaling pathway through TLR2 in OLs will be required to finely manipulate intracellular pro-survival machinery of OLs. Searching potent specific agonists either by utilizing endogenous ligands such as HMGB1 or newly identifying small molecules to selectively activate TLR2 in OLs without invoking proinflammatory activation in inflammatory cells will be future avenues for establishing the HMGB1/TLR2 as a novel therapeutic target for enhancing ischemic white matter restoration.
Figures
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
References
- Go to
- Abstract
- INTRODUCTION
- CLINICAL SPECTRUM OF ISCHEMIC WHITE MATTER DAMAGE
- ANIMAL MODELS OF ISCHEMIC WHITE MATTER DAMAGE
- RECENT ADVANCES IN IDENTIFYING CELLULAR AND MOLECULAR MECHANISMS OF ISCHEMIC WHITE MATTER INJURY
- TOLL-LIKE RECEPTOR 2 (TLR2) IN ISCHEMIC OL DEATH AND DEMYELINATION
- HIGH-MOBILITY GROUP BOX-1 (HMGB1) IS AN ENDOGENOUS TLR2 LIGAND AND PLAYS A ROLE OF AN AUTOCRINE TROPHIC FACTOR IN ISCHEMIC OL DEATH AND WHITE MATTER INJURY
- CONCLUSIONS AND FUTURE PERSPECTIVES
- Figure
- Reference
- Fields RD. White matter matters. Sci Am 2008;298:42-49.
- Hughes PM, Anthony DC, Ruddin M, Botham MS, Rankine EL, Sablone M, Baumann D, Mir AK, Perry VH. Focal lesions in the rat central nervous system induced by endothelin-1. J Neuropathol Exp Neurol 2003;62:1276-1286.
- Sozmen EG, Hinman JD, Carmichael ST. Models that matter: white matter stroke models. Neurotherapeutics 2012;9:349-358.
- Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010;10:826-837.
- Lin Q, Li M, Fang D, Fang J, Su SB. The essential roles of Toll-like receptor signaling pathways in sterile inflammatory diseases. Int Immunopharmacol 2011;11:1422-1432.
- Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci 2011;34:269-281.
- Gesuete R, Kohama SG, Stenzel-Poore MP. Toll-like receptors and ischemic brain injury. J Neuropathol Exp Neurol 2014;73:378-386.
- van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res 2009;175:139-148.
- Choi JY, Cui Y, Kang YM, Kim JH, Lee SJ, Kim BG. Role of toll-like receptor 2 in ischemic demyelination and oligodendrocyte death. Neurobiol Aging 2014;35:1643-1653.
- Choi JY, Cui Y, Chowdhury ST, Kim BG. High-mobility group box-1 as an autocrine trophic factor in white matter stroke. Proc Natl Acad Sci U S A 2017;114:E4987-E4995.
- Caplan LR. Lacunar infarction and small vessel disease: pathology and pathophysiology. J Stroke 2015;17:2-6.
- O'Sullivan M. Leukoaraiosis. Pract Neurol 2008;8:26-38.
- Roh JH, Lee JH. Recent updates on subcortical ischemic vascular dementia. J Stroke 2014;16:18-26.
- Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, Szaflarski J, Gebel J, Khoury J, Shukla R, Moomaw C, Pancioli A, Jauch E, Broderick J. Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke 2004;35:1552-1556.
- Ho PW, Reutens DC, Phan TG, Wright PM, Markus R, Indra I, Young D, Donnan GA. Is white matter involved in patients entered into typical trials of neuroprotection?. Stroke 2005;36:2742-2744.
- Leonards CO, Ipsen N, Malzahn U, Fiebach JB, Endres M, Ebinger M. White matter lesion severity in mild acute ischemic stroke patients and functional outcome after 1 year. Stroke 2012;43:3046-3051.
- Gladstone DJ, Black SE, Hakim AM, Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 2002;33:2123-2136.
- Hachinski VC, Potter P, Merskey H. Leuko-araiosis: an ancient term for a new problem. Can J Neurol Sci 1986;13:533-534.
- Moody DM, Bell MA, Challa VR. Features of the cere bral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol 1990;11:431-439.
- Pantoni L, Garcia JH, Gutierrez JA, Rosenblum WI. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996;27:1641-1646.
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689-701.
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652-659.
- Jokinen H, Gouw AA, Madureira S, Ylikoski R, van Straaten EC, van der Flier WM, Barkhof F, Scheltens P, Fazekas F, Schmidt R, Verdelho A, Ferro JM, Pantoni L, Inzitari D, Erkinjuntti T, LADIS Study Group. Incident lacunes influence cognitive decline: the LADIS study. Neurology 2011;76:1872-1878.
- Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, O'brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D. Impact of age-related cerebral white matter changes on the transition to disability -- the LADIS study: rationale, design and methodology. Neuroepidemiology 2005;24:51-62.
- Pantoni L, Poggesi A, Basile AM, Pracucci G, Barkhof F, Chabriat H, Erkinjuntti T, Ferro JM, Hennerici M, O'Brien J, Schmidt R, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D, LADIS Study Group. Leukoaraiosis predicts hidden global functioning impairment in nondisabled older people: the LADIS (Leukoaraiosis and Disability in the Elderly) Study. J Am Geriatr Soc 2006;54:1095-1101.
- Poggesi A, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Verdelho A, Hennerici M, Langhorne P, O'Brien J, Scheltens P, Visser MC, Crisby M, Waldemar G, Wallin A, Inzitari D, Pantoni L, Leukoaraiosis And DISability Study Group. Urinary complaints in nondisabled elderly people with age-related white matter changes: the Leukoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc 2008;56:1638-1643.
- Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke 1986;17:1090-1097.
- Kertesz A, Black SE, Tokar G, Benke T, Carr T, Nicholson L. Periventricular and subcortical hyperintensities on magnetic resonance imaging. ‘Rims, caps, and unidentified bright objects’. Arch Neurol 1988;45:404-408.
- Hachinski VC, Bowler JV, Loeb C. Vascular dementia. Neurology 1993;43:2159-2160.
- Akatsu H, Takahashi M, Matsukawa N, Ishikawa Y, Kondo N, Sato T, Nakazawa H, Yamada T, Okada H, Yamamoto T, Kosaka K. Subtype analysis of neuropathologically diagnosed patients in a Japanese geriatric hospital. J Neurol Sci 2002;196:63-69.
- Seno H, Ishino H, Inagaki T, Iijima M, Kaku K, Inata T, Hirai M. A neuropathological study of dementia in nursing homes in Shimane prefecture, Japan: evaluation of the age and gender effect. J Gerontol A Biol Sci Med Sci 1999;54:M312-M314.
- White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis 2009;18:713-725.
- Jellinger KA. Significance of brain lesions in Parkinson disease dementia and Lewy body dementia. Front Neurol Neurosci 2009;24:114-125.
- Wallin A, Milos V, Sjögren M, Pantoni L, Erkinjuntti T. Classification and subtypes of vascular dementia. Int Psychogeriatr. l 2003;15:27-37.
- Lee JH, Kim SH, Kim GH, Seo SW, Park HK, Oh SJ, Kim JS, Cheong HK, Na DL. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology 2011;77:18-25.
- Román GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol 2002;1:426-436.
- Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D, Array. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227-276.
- Schwartz GL, Fornage M, Mosley T, Turner ST. Treatment of leukoaraiosis. Curr Treat Options Cardiovasc Med 2005;7:173-177.
- Erkinjuntti T, Román G, Gauthier S, Feldman H, Rockwood K. Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke 2004;35:1010-1017.
- Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J Neurochem 2010;115:814-828.
- Du SQ, Wang XR, Xiao LY, Tu JF, Zhu W, He T, Liu CZ. Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion?. Mol Neurobiol 2017;54:3670-3682.
- Frost SB, Barbay S, Mumert ML, Stowe AM, Nudo RJ. An animal model of capsular infarct: endothelin-1 injections in the rat. Behav Brain Res 2006;169:206-211.
- Horie N, Maag AL, Hamilton SA, Shichinohe H, Bliss TM, Steinberg GK. Mouse model of focal cerebral ischemia using endothelin-1. J Neurosci Methods 2008;173:286-290.
- Hayakawa K, Pham LD, Seo JH, Miyamoto N, Maki T, Terasaki Y, Sakadžić S, Boas D, van Leyen K, Waeber C, Kim KW, Arai K, Lo EH. CD200 restrains macrophage attack on oligodendrocyte precursors via toll-like receptor 4 downregulation. J Cereb Blood Flow Metab 2016;36:781-793.
- Sozmen EG, Kolekar A, Havton LA, Carmichael ST. A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates. J Neurosci Methods 2009;180:261-272.
- Gadea A, Aguirre A, Haydar TF, Gallo V. Endothelin-1 regulates oligodendrocyte development. J Neurosci 2009;29:10047-10062.
- Hammond TR, Gadea A, Dupree J, Kerninon C, Nait-Oumesmar B, Aguirre A, Gallo V. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron 2014;81:588-602.
- Rosenzweig S, Carmichael ST. Age-dependent exacerbation of white matter stroke outcomes: a role for oxidative damage and inflammatory mediators. Stroke 2013;44:2579-2586.
- Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 2007;54:162-180.
- Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke 2004;35:2598-2603.
- Shibata M, Yamasaki N, Miyakawa T, Kalaria RN, Fujita Y, Ohtani R, Ihara M, Takahashi R, Tomimoto H. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke 2007;38:2826-2832.
- Hattori Y, Enmi J, Iguchi S, Saito S, Yamamoto Y, Tsuji M, Nagatsuka K, Kalaria RN, Iida H, Ihara M. Gradual carotid artery stenosis in mice closely replicates hypoperfusive vascular dementia in humans. J Am Heart Assoc 2016;5:e002757.
- Hattori Y, Enmi J, Kitamura A, Yamamoto Y, Saito S, Takahashi Y, Iguchi S, Tsuji M, Yamahara K, Nagatsuka K, Iida H, Ihara M. A novel mouse model of subcortical infarcts with dementia. J Neurosci 2015;35:3915-3928.
- Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology 2008;71:804-811.
- Révész T, Hawkins CP, du Boulay EP, Barnard RO, McDonald WI. Pathological findings correlated with magnetic resonance imaging in subcortical arteriosclerotic encephalopathy (Binswanger's disease). J Neurol Neurosurg Psychiatry 1989;52:1337-1344.
- Yamanouchi H. Loss of white matter oligodendrocytes and astrocytes in progressive subcortical vascular encephalopathy of Binswanger type. Acta Neurol Scand 1991;83:301-305.
- Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, Kim KW, Lo EH, Arai K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest 2013;123:782-786.
- Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M, Arai K. Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J Neurosci 2015;35:14002-14008.
- Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, Mandeville JB, Kim KW, Lo EH, Arai K. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke 2013;44:3516-3521.
- Sozmen EG, Rosenzweig S, Llorente IL, DiTullio DJ, Machnicki M, Vinters HV, Havton LA, Giger RJ, Hinman JD, Carmichael ST. Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. Proc Natl Acad Sci U S A 2016;113:E8453-E8462.
- Otero-Ortega L, Gutiérrez-Fernández M, Ramos-Cejudo J, Rodríguez-Frutos B, Fuentes B, Sobrino T, Hernanz TN, Campos F, López JA, Cerdán S, Vázquez J, Díez-Tejedor E. White matter injury restoration after stem cell administration in subcortical ischemic stroke. Stem Cell Res Ther 2015;6:121.
- Ramos-Cejudo J, Gutiérrez-Fernández M, Otero-Ortega L, Rodríguez-Frutos B, Fuentes B, Vallejo-Cremades MT, Hernanz TN, Cerdán S, Díez-Tejedor E. Brain-derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke 2015;46:221-228.
- Otero-Ortega L, Laso-García F, Gómez-de Frutos MD, Rodríguez-Frutos B, Pascual-Guerra J, Fuentes B, Díez-Tejedor E, Gutiérrez-Fernández M. White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci Rep 2017;7:44433.
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptormediated neuronal injury. Glia 2010;58:253-263.
- Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine 2010;49:1-9.
- Shintani Y, Kapoor A, Kaneko M, Smolenski RT, D'Acquisto F, Coppen SR, Harada-Shoji N, Lee HJ, Thiemermann C, Takashima S, Yashiro K, Suzuki K. TLR9 mediates cellular protection by modulating energy metabolism in cardiomyocytes and neurons. Proc Natl Acad Sci U S A 2013;110:5109-5114.
- Bsibsi M, Nomden A, van Noort JM, Baron W. Toll-like receptors 2 and 3 agonists differentially affect oligodendrocyte survival, differentiation, and myelin membrane formation. J Neurosci Res 2012;90:388-398.
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 2002;61:1013-1021.
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci 2002;22:2478-2486.
- Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A 2010;107:11555-11560.



