Articles
Article Tools
Stats or Metrics
Article
Technologue
Exp Neurobiol 2018; 27(1): 57-64
Published online February 28, 2018
https://doi.org/10.5607/en.2018.27.1.57
© The Korean Society for Brain and Neural Sciences
Development of Electrical Neural Stimulator Generating Periodic and Non-periodic Signals and Supporting Closed-loop Experimental System
Hyejin An and Hyun-Chool Shin*
Department of Electronic Engineering, Soongsil University, Seoul 06978, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-828-7165, FAX: 82-2-821-7653
e-mail: shinhc@ssu.ac.kr
Abstract
It is essential to build a system to generate proper neural stimulus signals with adjusting parameters. We developed a stimulator with up to four channels for separate settings in periodic and non-periodic modes. The device can support a closed-loop experimental system which utilizes neural information in real time as a feedback for controlling stimuli. To confirm whether stimulating signals are properly produced and delivered inside the brain, two experiments with rats were conducted. We observed that the change of firing rates and the cross-power spectral density increased after stimulation which meant that electric signals were transferred well and that they affected the neurons' activities. Thus, it is expected that the stimulator can be utilized to produce appropriate stimulation signals in accordance with various objectives.
Graphical Abstract
Keywords: stimulator, brain stimulation, electrical stimulation, neuron, closed-loop system
INTRODUCTION
Brain diseases due to damages in the nervous system may lead to a number of sequelae such as dysphrasia or dyskinesia. These illnesses can be commonly found since they include depression, posttraumatic stress disorder, Parkinson's disease, and Alzheimer's disease. In the past, most treatments involved drugs or psychological counseling, but they were not effective in all patients. To solve this problem, the development of treatment methods using brain stimulation was attempted [1,2,3,4,5,6,7] and the corresponding studies advanced quite actively not only for the treatment itself but also for the brain stimulation to improve the brain functions [8,9].
Brain-stimulating methods can be categorized into two types: (1) non-invasive brain stimulation which stimulates the surface of the head such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) and (2) the invasive type that stimulates the brain by connecting a device inside it, such as deep brain stimulation (DBS) or optogenetics [10,11]. Among them, DBS is a method in which a stimulating device is transplanted in the brain to stimulate neurons directly with an electric stimulus. This stimulates a local area in need of a stimulus and, at the same time, has the advantage of minimizing the stimulation to the areas surrounding the brain. DBS can control the stimulation while keeping the original brain structure undamaged. Therefore, various signals can be set according to the personal states of patients and the stimulation can be controlled by the current condition of each individual in real time. Using these features of DBS, many researchers have employed it in nerve-related disease treatments such as Parkinson's disease, dystonia, Tourette syndrome and studied the method with regard to learning capacity and strengthening memory [12,13,14,15].
Due to the wide use of stimuli, it must be possible to adjust the stimulating areas, proper electric signal types, and waveforms. The signal parameters for DBS include, among others, the pulse width, the frequency, and the amplitude. To simultaneously achieve the maximization of DBS efficiency and the minimization of adverse side effects, it is necessary to establish an environment in which stimulating variables can be set in both various and specific ways. If an excessive electric current is delivered to the brain, it may cause the destruction of neurons and side effects such as epilepsy. To prevent such risks, a number of researchers combined each parameter in various methods to conduct experiments with the aim of finding stimulating signals appropriate for their purposes [16,17].
If appropriate stimulating signals are set and the stimulus reflects the response from the neurons as a feedback, an even greater DBS effect may be produced. To do this, studies on DBS incorporating a closed-loop system have been frequently performed recently. Salam et al. [18] conducted an experiment that compared the differences in inhibiting seizures by the assignment of open-loop and closed-loop stimuli to the hippocampus of epilepsy model rats, proving that the closed-loop stimulus had a better effect. Rosin et al. [19] stimulated the globus pallidum of African green monkeys and reported that the closed-loop stimulus was more effective in improving Parkinson's disease.
To improve the performance of DBS, we developed a brain stimulator which has all four functions: (1) to select periodic or nonperiodic modes (2) to specify the obvious time stamps of pulses in non-periodic mode (3) to control up to four channels independently (4) to build the closed-loop environment. The device can produce various stimulating signals and control the electric stimulus according to the firing information of neurons in a real-time closed-loop environment. Such a system supports the function of non-periodic mode setting, which helps in finding appropriate stimulating signals by generating stimulating signals acquired by actual neural activities. Up to four channels can be set in periodic or non-periodic modes and the stimulating signal variables can be adjusted independently in this device. Since this developed stimulator can produce diverse types of stimuli, it can be used in various experiments.
MATERIALS AND METHODS
Description of the developed stimulator
Stimulator functions
The brain stimulator generates stimulating signals through up to four channels and is capable to independently set the activation of each channel, periodic/non-periodic modes, and the signal type. The signal types, the pulse width, the period, and the number of repetitions can be designated within a given range. The stimulator can be conveniently controlled by a software control panel that interacts with the device. Although the periodic mode can simply adjust stimulating parameters, it has limited ranges of parameters due to a hardware restriction. The non-periodic mode solves the problem by directly entering pulse time stamps. Therefore, to select a proper mode helps produce stimulus signals in various ways.
There are two methods to start and stop the assignment of stimulating signals: The first one is a simple method to push a start/stop button to control; the second way is to set trigger conditions and then the stimulating signals are generated or terminated if any one of the user-defined conditions is satisfied. With regard to the trigger conditions, either the sensor installed in the experimental device or the neuron firing signals in the animal subjects' brains can be used.
Stimulator board
Fig. 1 describes a stimulator board (20 cm×15 cm). It receives the stimulating signal information sent by a software program in a USB part. By the data, the device generates the signals in a field programmable gate array (FPGA) and a clock generator. The board transfers the stimulating signals through digital-to-analog (DAC) converter and Byonet Neill-Concelman (BNC) connectors. Four DAC and BNC connectors are included and up to four channels can be used.
Software control panel
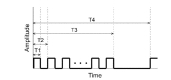
Fig. 2 displays the main window screen of the stimulator's software control panel and Fig. 3 shows the magnified part that sets the stimulating signals in Fig. 2. As in Fig. 4, T1 is the pulse width, T2 the single pulse period, and T3 the width of one epoch. T4 indicates the time required from the start of one epoch to the transfer of the next, and “Repeat” refers to the number of repeats in the T4 interval. Amplitude is the size of the stimulating signal. Table 1 exhibits the designated ranges of these six parameters. A number of stimulating signal types can be generated while adjusting the input values minutely by combining these parameters.
The periodic and non-periodic modes are set in the area of Fig. 3. If the non-periodic mode is selected, the T2 and T3 entries will be terminated and, at the same time, the file-open button will be activated. If the text file that stores stimulus signals is loaded after this button is clicked, those instead of T2 and T3 will be reflected in the stimulation signals. In other words, the stimulator generates pulse signals with the width of T1 stored in the text file.
The stimulator can support two other functions in addition to the basic one for stimulus generation. However, to use these functions, an environment is required in which the network between the stimulator software program and experimental data acquisition system is connected. The stimulator provides the function to receive log messages such as neuron signals, sensors, and certain events from the data acquisition system. These messages can be monitored in real time and they are automatically saved in a text file. The other function serves to conduct the closed-loop experiment, which controls the stimulating signal assignment automatically if the log message and the trigger conditions are set in advance.
RESULTS
Composition of experimental devices
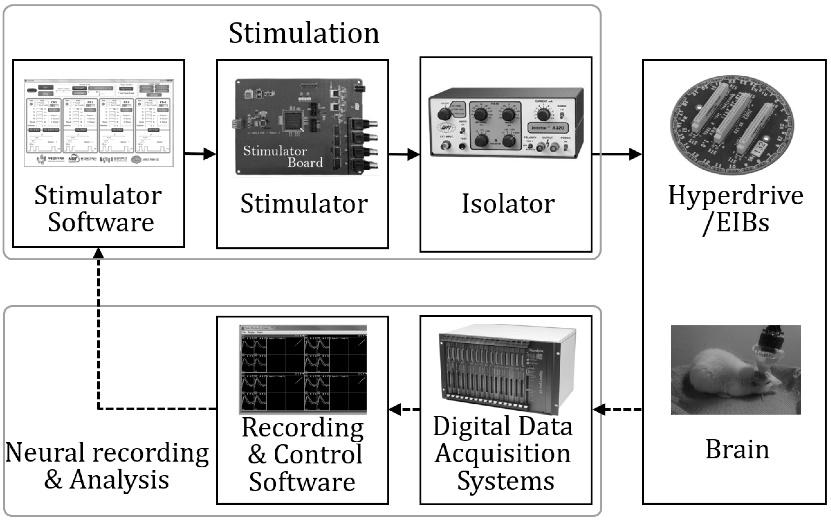
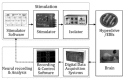
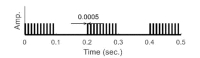
Fig. 5 illustrates that the closed-loop experimental devices are composed of stimulation, neural recording, and analysis parts. After the establishment of a desired stimulating signal through the software control panel, this information is transferred to the stimulator board. The signals generated in the board are transferred to the isolator and pass a BNC connector which is the output part of the board. The isolator prevents a reverse current and serves as a setter of the current's magnitude. The signals that pass through the isolator are delivered to the brain through a hyperdrive which includes electrode interface boards (EIBs). At the same time, the digital data acquisition system receives the neuron information of the brain delivered through the hyperdrive. This information is saved in the computer by the software program which is compatible with the acquisition system. The system and the program do not only possess the functions to transfer and save the data, but also to control the experimental devices. The stimulator program receives the log message from the acquisition system program and delivers the electric stimulus according to the trigger condition. In this paper, we use firing information of a specific cell as the trigger condition of the closed-loop system. The data were extracted from neural information by the recording and control program. Fig. 6 is a photograph that shows the actual stimulation experimental environment and Fig. 7 depicts the output screen of the oscilloscope connected to the isolator's output part. The pulse with 1 msec. of width is generated at every second.
Experiment I
To identify whether the signals generated in the stimulator are properly delivered, an experiment was performed in which the stimulus was directly administered to the brain of the rats. All protocols conformed to the Institutional Animal Care and Use Committee of the Seoul National University. Eight tetrodes were transplanted in the dorsal hippocampus and sixteen in the ventral hippocampus to record the signals of the neurons. The stimulated area was the basolateral amygdala (BLA) near the hippocampus. The hippocampus plays an important role in memory and learning, while the BLA is related to fear. The theta burst stimulation (TBS) was assigned for 1 sec at which the pulse width was 500 µsec., the pulse frequency 100 Hz, and the burst epoch frequency 10 Hz (Fig. 8).
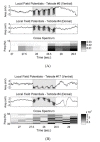
Fig. 9 describes whether the stimulating signals are properly transferred to neurons and whether the stimuli affect the neurons. The local field potential (LFP) is the electrical potential generated in the neurons near the tetrode. The gray interval in the LFP shows the interval at which the stimulus is entered. The potentials in the interval reflect the stimulating signals.
In addition, the cross-power spectrum between two tetrodes was examined with regard to changes in the relationship between the neurons of the ventral hippocampus and the ones in the dorsal hippocampus caused by the stimulus. When the signals
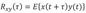
where
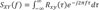
where ƒ is the frequency.
To understand the CPSD, it was calculated after dividing the frequency domain of LFP into the theta band (4~12 Hz) and the gamma band (30-Hz). It is known that the theta rhythm is related with memory including spatial memory information [20,21], and the gamma rhythm with recall or working memory [22,23]. In each figure below Fig. 9 (A) and (B), the value of the cross spectrum increased after receiving stimulation, indicating that the electric stimulus had a slight effect on the neurons.
Experiment II
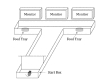
The second experiment was conducted in a T-maze (Fig. 10) to observe what effect the stimulus had on the behavior of rats or the neurons' activities. As the door of the start box opened, the animals saw the image on the monitor and then found the tray which contained food. The trials with and without the electric stimulus proceeded randomly at the probability of 50%. In the trial with the stimulus, the rats were stimulated while they passed a linear course after the start box opened. On the first day of the experiment, we used sensors attached to the T-maze as the trigger conditions for the start and end time of the stimulation. On the second day, the firing information in a certain cell of the rat brain was used instead of the sensors to perform the experiment in the closed-loop environment. The information played a role on a trigger to control start/stop signs in a real-time. The stimulator took the neural information and delivered electric stimulus. The answer selection was either the left or the right according to the image and if the rats found the answer, they could eat cereals in the food tray as a reward. The details of the experiment protocol can be found in [24]. For stimulating and recording, a 24-tetrode hyperdrive was implanted in area CA1 of the hippocampus.
This experiment was conducted for two days. On the first day, the theta rhythm's stimulus with the single pulse frequency of 7 Hz and the single pulse width of 1 msec. was administered with three volts, as in (A) of Fig. 11. On the second day, the single pulse frequency was 100 Hz, the pulse width 1 msec., and the burst epoch frequency 10 Hz, as in (B) of Fig. 11. The voltage was 2.3 volts and it was designed not to exceed a maximum of 2 sec. when entered. Although the signals shown in Fig. 11 (A) are periodic, they cannot be set to the periodic mode due to a limit condition of the input parameter ranges. To solve this problem, we set the non-periodic mode and used the text file with the time points of the pulses. In other words, the software panel could overcome the hardware limit of the periodic mode by using the non-periodic mode instead.
Fig. 12 shows the correct rates of the trials with (black bar) and without the stimulus (white bar). A single session consisted of about 40 trials. Three sessions were conducted on the first day and four sessions on the second day. As the experiment proceeded, it was found that the correct rate significantly declined in the trial with the stimulus compared to the trial without it. This result was observed in both experiments on both days. It was supposed that the stimulating signals affected the rat behavior by the difference of correct rates between trials with stimulus and without stimulus in each latter part of both experiments.
Fig. 13 displays the spike raster before and after the starting point of the stimulus assignment in certain neurons and a peristimulus time histogram (PSTH). The line in the center is the time point where stimulus began, containing about 1 sec. of information with 0.5 sec. each before and after stimulation. The spike raster describes every firing time with dots after the trials in the absence or the presence of the stimulus. The PSTH describes the firing rate of neurons per unit time, expressed in a block window with the bin size of 0.05 sec and the number in it is the absolute contrast. After calculating the absolute value of the number of spikes' difference in the front and the rare intervals based on the event time, the result was then divided by the summed number of spikes as shown in formula (3), where c is the absolute contrast, 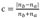
If the number of spikes is identical, the absolute contrast would be 0 and if there is a firing in one interval before or after the stimulation, it would be 1. The variation is proportional to the absolute contrast by the equation. In case of a trial with stimulation, the absolute contrast is higher, which means that the firing rate before and after the firing time changed considerably.
DISCUSSION
DBS is a technique used to treat various brain diseases and to improve the brain functions which aims to minimize side effects and to maximize the therapeutic outcome. Therefore, it is the key for various parameter adjustments of stimulating signals to administer an appropriate stimulus. Since the proposed stimulator provides not only a periodic mode but also a non-periodic one, it can generate a variety of stimulating signals. In addition, as the condition of neurons changes every minute, the effects of DBS will be even greater if real-time feedback is provided. This stimulator has function to reflect the signals of neurons in real time through the closed-loop system and to generate stimuli. Although there is a conceivable limit in improving or investigating brain functions through experimental results, it was determined that the stimulating signals were well delivered to the neurons of the brain and that the signals affected the neurons' firing. This stimulator is expected to be useful for future DBS-related research.
Figures








Tables
| Periodic | Non-Periodic | |
|---|---|---|
| T1 (µs) | 100–1,000 | 100–1,000 |
| T2 (ms) | 2–1,000 | User-designed sequence |
| T3 (ms) | 2–10,000 | User-designed sequence |
| T4 (s) | 1–60 | 1–60 |
| Repetitions | 1–60 | 1–60 |
| Amplitude (V) | 0.1–5 | 0.1–5 |
(T1<T2<T3<T4).
References
- Karsen EF, Watts BV, Holtzheimer PE. Review of the effectiveness of transcranial magnetic stimulation for post-traumatic stress disorder. Brain Stimul 2014;7:151-157.
- Ngoga D, Mitchell R, Kausar J, Hodson J, Harries A, Pall H. Deep brain stimulation improves survival in severe Parkinson's disease. J Neurol Neurosurg Psychiatry 2014;85:17-22.
- Hsu WY, Ku Y, Zanto TP, Gazzaley A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer's disease: a systematic review and meta-analysis. Neurobiol Aging 2015;36:2348-2359.
- Raffin E, Siebner HR. Transcranial brain stimulation to promote functional recovery after stroke. Curr Opin Neurol 2014;27:54-60.
- Hasan A, Wobrock T, Rajji T, Malchow B, Daskalakis ZJ. Modulating neural plasticity with non-invasive brain stimulation in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2013;263:621-631.
- Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry 2013;73:1204-1212.
- Stidd DA, Vogelsang K, Krahl SE, Langevin JP, Fellous JM. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain Stimul 2013;6:837-844.
- Flöel A, Rösser N, Michka O, Knecht S, Breitenstein C. Noninvasive brain stimulation improves language learning. J Cogn Neurosci 2008;20:1415-1422.
- Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, Fried I. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med 2012;366:502-510.
- Kang N, Summers JJ, Cauraugh JH. Non-invasive brain stimulation improves paretic limb force production: a systematic review and meta-analysis. Brain Stimul 2016;9:662-670.
- Lewis PM, Thomson RH, Rosenfeld JV, Fitzgerald PB. Brain neuromodulation techniques: a review. Neuroscientist 2016;22:406-421.
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, Starr PA. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci 2015;18:779-786.
- Toda H, Saiki H, Nishida N, Iwasaki K. Update on deep brain stimulation for dyskinesia and dystonia: a literature review. Neurol Med Chir (Tokyo) 2016;56:236-248.
- Molina R, Okun MS, Shute JB, Opri E, Rossi PJ, Martinez-Ramirez D, Foote KD, Gunduz A. Report of a patient undergoing chronic responsive deep brain stimulation for Tourette syndrome: proof of concept. J Neurosurg 2017.
- Suthana N, Fried I. Deep brain stimulation for enhancement of learning and memory. Neuroimage 2014;85 Pt 3:996-1002.
- Hescham S, Lim LW, Jahanshahi A, Steinbusch HW, Prickaerts J, Blokland A, Temel Y. Deep brain stimulation of the forniceal area enhances memory functions in experimental dementia: the role of stimulation parameters. Brain Stimul 2013;6:72-77.
- Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clin Neurophysiol 2004;115:2431-2441.
- Salam MT, Velazquez JL, Genov R. Seizure suppression efficacy of closed-loop versus open-loop deep brain stimulation in a rodent model of epilepsy. IEEE Trans Neural Syst Rehabil Eng 2016;24:710-719.
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 2011;72:370-384.
- Buzsáki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 2005;15:827-840.
- Chauvière L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci 2009;29:5402-5410.
- Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci 2004;8:347-355.
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex 2003;13:1369-1374.
- Kim S, Lee J, Lee I. The hippocampus is required for visually cued contextual response selection, but not for visual discrimination of contexts. Front Behav Neurosci 2012;6:66.



