Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2019; 28(1): 85-103
Published online January 21, 2019
https://doi.org/10.5607/en.2019.28.1.85
© The Korean Society for Brain and Neural Sciences
Restorative Mechanism of Neural Progenitor Cells Overexpressing Arginine Decarboxylase Genes Following Ischemic Injury
Jae Young Kim1, Jong Youl Kim1, Jae Hwan Kim1,4, Hosung Jung1,2, Won Taek Lee1, and Jong Eun Lee1,2,3*
1Department of Anatomy, Yonsei University College of Medicine, Seoul 03722, Korea.
2BK21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul 03722, Korea.
3Brain Research Institute, Yonsei University College of Medicine, Seoul 03722, Korea.
4Center for Neuroscience Imaging Research (CNIR), Institute for Basic Science, Sungkyunkwan University, Suwon 16419, Korea.
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-2228-1646, FAX: 82-2-365-0700
e-mail: jelee@yuhs.ac
Abstract
Cell replacement therapy using neural progenitor cells (NPCs) following ischemic stroke is a promising potential therapeutic strategy, but lacks efficacy for human central nervous system (CNS) therapeutics. In a previous
Graphical Abstract
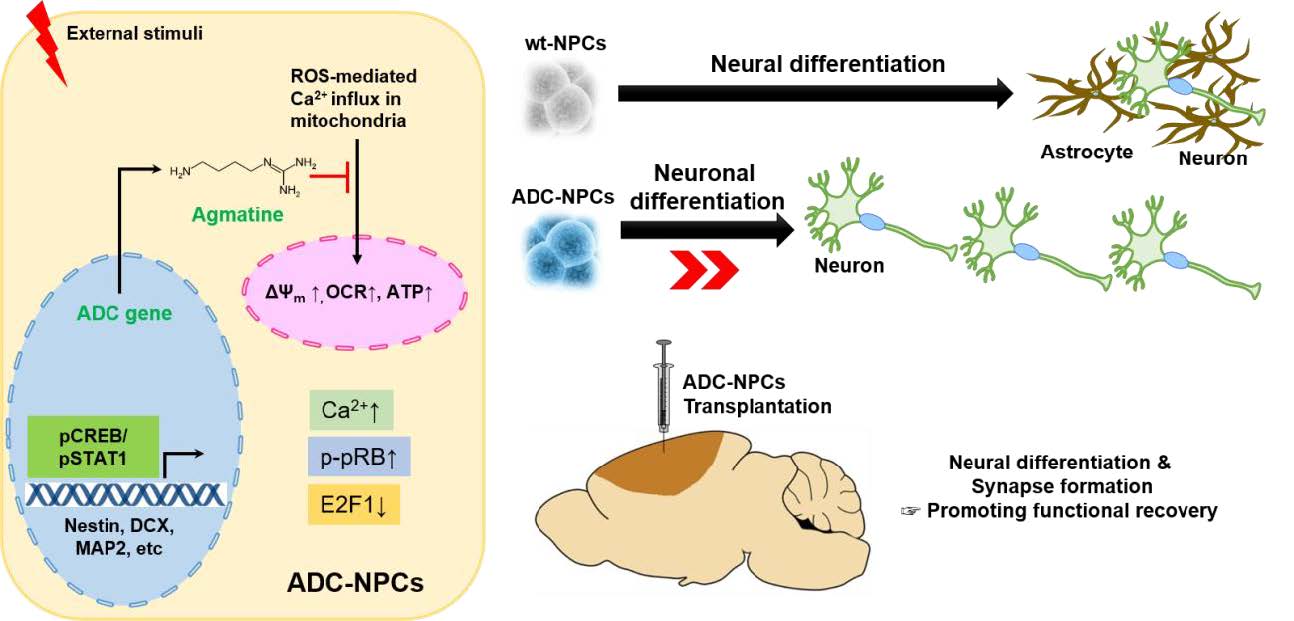
Keywords: Ischemic stroke, Cell replacement therapy, Neural progenitor cells, Arginine decarboxylase, Cell cycle arrest, Neural differentiation


