Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2020; 29(3): 230-248
Published online June 22, 2020
https://doi.org/10.5607/en20017
© The Korean Society for Brain and Neural Sciences
Absence of Glia Maturation Factor Protects from Axonal Injury and Motor Behavioral Impairments after Traumatic Brain Injury
Govindhasamy Pushpavathi Selvakumar1,2,3, Mohammad Ejaz Ahmed1,2,3, Shankar S. Iyer1,2,3, Ramasamy Thangavel1,2,3, Duraisamy Kempuraj1,2,3, Sudhanshu P. Raikwar1,2,3, Kieran Bazley2,3, Kristopher Wu2,3, Asher Khan2,3, Klaudia Kukulka2,3, Bret Bussinger2,3, Smita Zaheer2,3, Casey Burton4, Donald James4 and Asgar Zaheer1,2,3*
1Harry S. Truman Memorial Veterans Hospital, Columbia, Missouri 65211, 2Department of Neurology, and 3Center for Translational Neuroscience, School of Medicine, University of Missouri, Columbia, Missouri 65211, 4Phelps Health, Rolla, Missouri 65401, USA
Correspondence to: *To whom correspondence should be addressed.
TEL: 573-882-5386, FAX: 573-882-5386
e-mail: zaheera@health.missouri.edu
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License
(http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and
reproduction in any medium, provided the original work is properly cited.
Abstract
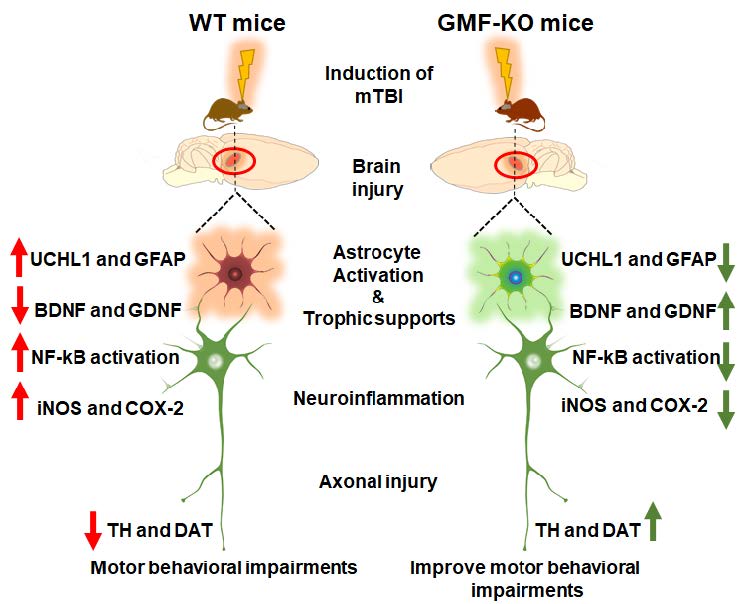
Traumatic brain injury (TBI) causes disability and death, accelerating the progression towards Alzheimer’s disease and Parkinson’s disease (PD). TBI causes serious motor and cognitive impairments, as seen in PD that arise during the period of the initial insult. However, this has been understudied relative to TBI induced neuroinflammation, motor and cognitive decline that progress towards PD. Neuronal ubiquitin-C-terminal hydrolase- L1 (UCHL1) is a thiol protease that breaks down ubiquitinated proteins and its level represents the severity of TBI. Previously, we demonstrated the molecular action of glia maturation factor (GMF); a proinflammatory protein in mediating neuroinflammation and neuronal loss. Here, we show that the weight drop method induced TBI neuropathology using behavioral tests, western blotting, and immunofluorescence techniques on sections from wild type (WT) and GMF-deficient (GMF-KO) mice. Results reveal a significant improvement in substantia nigral tyrosine hydroxylase and dopamine transporter expression with motor behavioral performance in GMF-KO mice following TBI. In addition, a significant reduction in neuroinflammation was manifested, as shown by activation of nuclear factor-kB, reduced levels of inducible nitric oxide synthase, and cyclooxygenase- 2 expressions. Likewise, neurotrophins including brain-derived neurotrophic factor and glial-derived neurotrophic factor were significantly improved in GMF-KO mice than WT 72 h post-TBI. Consistently, we found that TBI enhances GFAP and UCHL-1 expression and reduces the number of dopaminergic TH-positive neurons in WT compared to GMF-KO mice 72 h post-TBI. Interestingly, we observed a reduction of THpositive tanycytes in the median eminence of WT than GMF-KO mice. Overall, we found that absence of GMF significantly reversed these neuropathological events and improved behavioral outcome. This study provides evidence that PD-associated pathology progression can be initiated upon induction of TBI.
Graphical Abstract
Keywords: Traumatic brain injury, Neuroinflammation, Glia maturation factor, Parkinson’s disease, Motor behavior


