Articles
Article Tools
Supplementary
Stats or Metrics
Article
Original Article
Exp Neurobiol 2021; 30(4): 275-284
Published online August 31, 2021
https://doi.org/10.5607/en21012
© The Korean Society for Brain and Neural Sciences
Contribution of Extracellular Matrix Component Landscapes in the Adult Subventricular Zone to the Positioning of Neural Stem/Progenitor Cells
Hyun Jung Kim1,2†, Eunsoo Lee1,3†, Myungwoo Nam1, Jae Kwon Chung1, Sunghoon Joo4, Yoonkey Nam4 and Woong Sun1*
1Department of Anatomy, College of Medicine, Korea University, Seoul 02841, 2Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejon 34141, 3Fluorescence Core Imaging Center, Ewha Womans University, Seoul 03760, 4Department of Bio and Brain Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-2286-1404, FAX: 82-2-929-5696
e-mail: woongsun@korea.ac.kr
†These authors contributed equally to this work.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
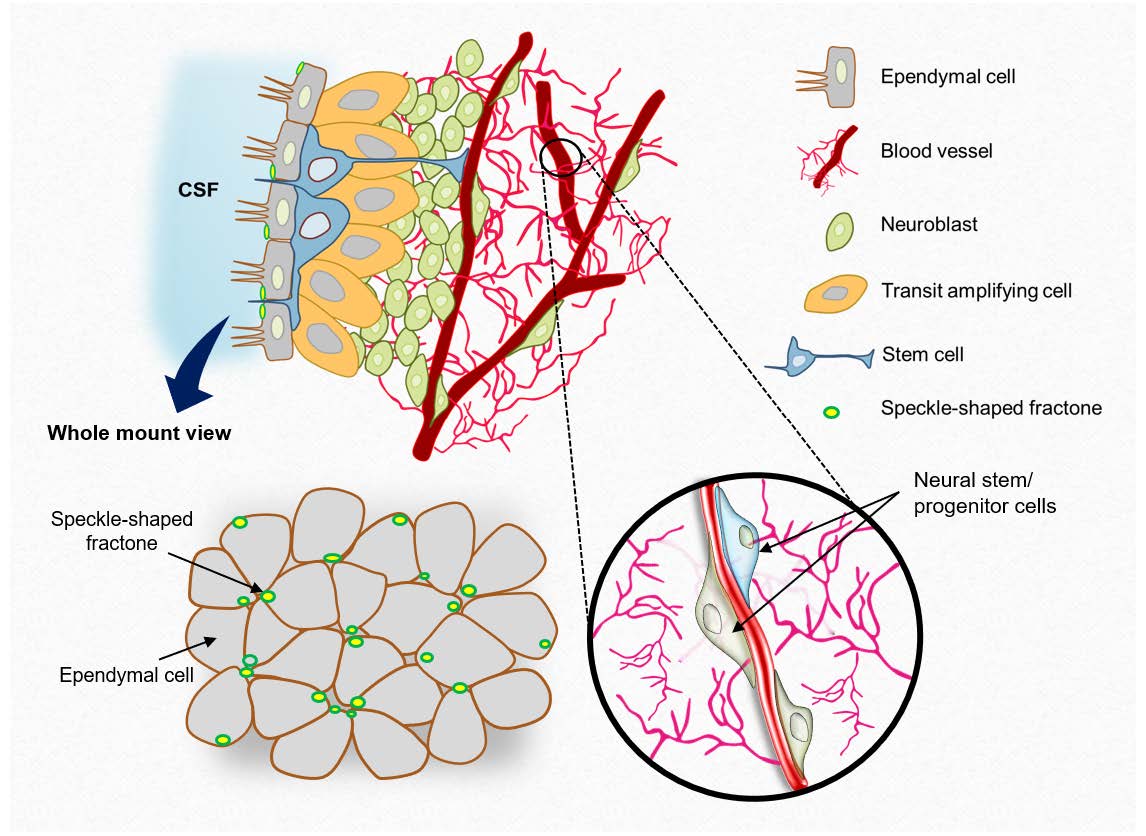
Neurogenesis persists in restricted regions of the adult brain, including the subventricular zone (SVZ). Adult neural stem cells (NSCs) in the SVZ proliferate and give rise to new neurons and glial cells depending on intrinsic and environmental cues. Among the multiple factors that contribute to the chemical, physical, and mechanical components of the neurogenic niche, we focused on the composition of the extracellular matrix (ECM) of vasculature and fractones in the SVZ. The SVZ consists of ECM-rich blood vessels and fractones during development and adulthood, and adult neural stem/progenitor cells (NS/PCs) preferentially attach to the laminin-rich basal lamina. To examine the ECM preference of adult NS/PCs, we designed a competition assay using cell micropatterning. Although both laminin and collagen type IV, which are the main components of basal lamina, act as physical scaffolds, adult NS/PCs preferred to adhere to laminin over collagen type IV. Interestingly, the ECM preference of adult NS/PCs could be manipulated by chemokines such as stromal-derived factor 1 (SDF1) and α6 integrin. As SDF1 re-routes NSCs and their progenitors toward the injury site after brain damage, these results suggest that the alteration in ECM preferences may provide a molecular basis for contextdependent NS/PC positioning.
Graphical Abstract
Keywords: ECM, Basal lamina, Fractone, Subventricular zone, Neural stem/progenitor cell


