Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2022; 31(5): 289-298
Published online October 31, 2022
https://doi.org/10.5607/en22001
© The Korean Society for Brain and Neural Sciences
Distribution of Neuroglobin in Pericytes is Associated with Blood-Brain Barrier Leakage against Cerebral Ischemia in Mice
Yeojin Kim1, Mingee Kim1, So-Dam Kim1, Naeun Yoon1, Xiaoying Wang2, Gyu-Un Bae1 and Yun Seon Song1*
1Department of Pharmacology, College of Pharmacy, Drug Information Research Institute, Muscle Physiome Research Center, Sookmyung Women’s University, Seoul 04310, Korea
2Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2- 2077-7231, FAX: 82-2-710-9871
e-mail: yssong@sookmyung.ac.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
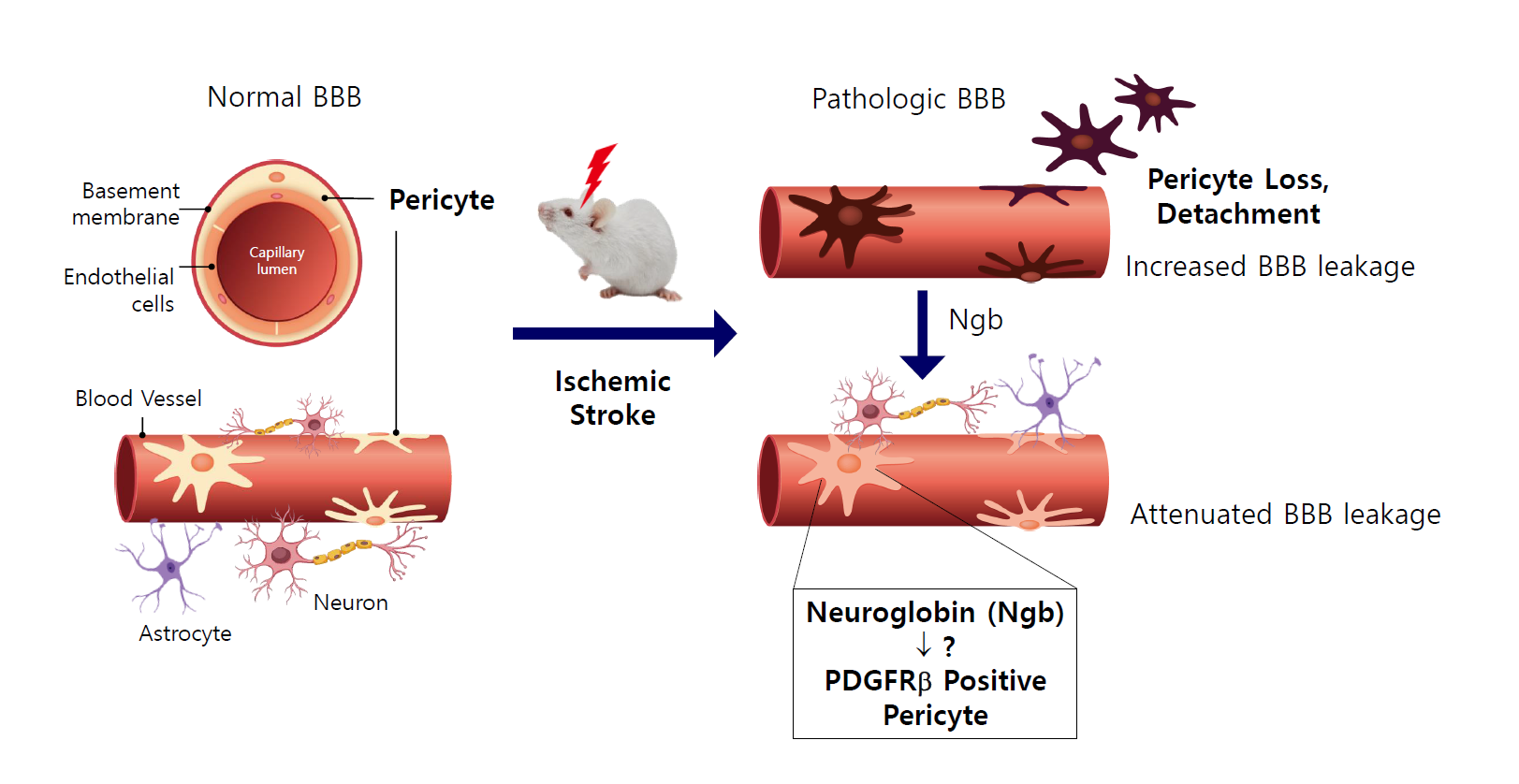
With emerging data on the various functions of neuroglobin (Ngb), such as neuroprotection and neurogenesis, we investigated the role of Ngb in the neurovascular unit (NVU) of the brain. To study the distribution and function of Ngb after cerebral ischemia, transient middle cerebral artery occlusion (tMCAO) was performed in mice. Brain immunostaining and fluorescence-activated cell sorting were used to analyze the role of Ngb according to the location and cell type. In normal brain tissue, it was observed that Ngb was distributed not only in neurons but also around the brain’s blood vessels. Interestingly, Ngb was largely expressed in platelet-derived growth factor receptor β (PDGFRβ)-positive pericytes in the NVU. After tMCAO, Ngb levels were significantly decreased in the core of the infarct, and Ngb and PDGFRβ-positive pericytes were detached from the vasculature. In contrast, in the penumbra of the infarct, PDGFRβ-positive pericytes expressing Ngb were increased compared with that in the core of the infarct. Moreover, the cerebral blood vessels, which have Ngb-positive PDGFRβ pericytes, showed reduced blood-brain barrier (BBB) leakage after tMCAO. It showed that Ngb-positive PDGFRβ pericytes stayed around the endothelial cells and reduced the BBB leakage in the NVU. Our results indicate that Ngb may play a role in attenuating BBB leakage in part by its association with PDGFRβ. In this study, the distribution and function of Ngb in the pericytes of the cerebrovascular system have been elucidated, which contributes to the treatment of stroke through a new function of Ngb.
Graphical Abstract
Keywords: Neuroglobin, Pericytes, Ischemic stroke, Blood-brain barrier
INTRODUCTION
Stroke is a cerebrovascular disease that causes death and disability worldwide. It affects the arteries leading to and within the brain, and blood-brain barrier (BBB) disruption following ischemic stroke can result in severe vasogenic edema formation and hemorrhagic transformation. The BBB does not function independently but as a module within the greater context of the multicellular neurovascular unit (NVU) [1]. The NVU comprises various vascular cells (pericytes, smooth muscle cells, endothelial cells), glial cells (astrocytes, microglia, oligodendrocytes), and neurons [2]. Regeneration of NVU components serves as a reconstructive mechanism in the neuropathology of brain diseases.
Pericytes are known to maintain the BBB in the brain and have diverse functions, especially during hypoxia; they rapidly change their anatomical morphology and function in sync with endothelial cells [3]. In response to ischemia, pericytes produce reactive oxygen species (ROS) since they are sensitive to low concentrations of ROS [4]. In hypoxic conditions, pericyte detachment increases instability in patients with arteriovenous malformation [5]. In contrast, migration of pericytes could serve as a protective mechanism to prevent death during injury [6]. It has been suggested that pericytes acquire the ability to differentiate into neuronal, microglial, and vascular cells after conditions such as ischemic diseases and hypoxia [7].
Pericytes may express a variety of factors in response to ischemia to protect adjacent cells, including neurons and other NVU components [4]. Among various factors, platelet-derived growth factor B (PDGF-B), expressed by endothelial cells, binds to platelet-derived growth factor receptor β (PDGFRβ) on pericytes and induces pericyte proliferation and survival. PDGF-B has been shown to significantly increase the expression of nerve growth factor and neurotrophin-3 (NT- 3) through Akt in pericytes [8]. Once PDGF-B binds to its receptor, receptor dimerization induces several signaling pathways that have been associated with migration, proliferation, and survival [9], but the underlying mechanisms remain poorly understood.
Neuroglobin (Ngb) is an endogenous neuroprotective molecule that is expressed predominantly in the brain. It is induced by neuronal hypoxia and cerebral ischemia, and it protects against hypoxic or ischemic neuronal injury [10]. Ngb enhances cell viability under hypoxia and various types of oxidative stress in transgenic systems [11]. It is thought to transport oxygen across the BBB and/or interfere with the intrinsic pathway of apoptosis in mitochondria [12]. A newly discovered function of Ngb is that it promotes neurogenesis in mice after stroke [13]. Neurogenesis, which is the formation of new neurons by neural stem/progenitor cells (NSPCs), occurs throughout life as well as in pathological conditions of the brain [14]. Reports indicate that Ngb may affect neurons as well as pericytes, which have developmental (differentiation) potential in the BBB.
Since pericytes play important roles under physiological conditions and mediate vital pathological processes in ischemic stroke, there is an upsurge of interest in developing therapeutic strategies for ischemic stroke targeting pericytes.
Considering emerging evidence that the role of Ngb in neurogenesis promotes NSPC differentiation into various brain cells, we expected Ngb to exist in neurons and other brain cell types. In the present study, we investigated whether Ngb exists in vascular lineage cells, pericytes in NVU, and the role of NVU and BBB integrity after transient middle cerebral artery occlusion (tMCAO) in mice.
MATERIALS AND METHODS
All animal experiments were performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care of Sookmyung Women’s University. After the experiments, all animals were euthanized with an overdose of anesthetic to minimize pain or discomfort.
Animals
Male C57BL/6 mice (21~25 g, 10~12 weeks) were used in this experiment (Orient, Seongnam, South Korea). The animals were housed in a purpose-built facility with a controlled environment and maintained in an isolator set to maintain temperature and relative humidity at 24±2℃ and 50%, respectively. Artificial lighting provided a 24 h cycle of 12 h light:12 h dark. Sterile water and food were also supplied.
Transient middle cerebral artery occlusion (tMCAO)
Female mice were subjected to tMCAO and reperfusion for 45 min. Transient focal cerebral ischemia was induced in the mice by occlusion of the left MCA using a 5-0 suture (monofilament nylon). Briefly, the left common carotid artery was exposed, and the external carotid artery (ECA) was dissected distally. The internal carotid artery (ICA) was isolated, and a blunted suture was introduced into the ECA lumen and then gently advanced into the ICA lumen to block MCA blood flow. After 45 min of tMCAO, cerebral blood flow was restored by suture withdrawal. The incision was closed, and the mice were allowed to recover on a heating pad.
Cresyl violet staining
After MCAO, the brains were excised, frozen for 1 h at -80℃, and serially cryosectioned. Cresyl violet staining is generally used to quantify experimental brain infarcts. The sections were rehydrated using a sequence of ethanol baths (50, 70, 80, 90, and 100%) and immersed in distilled water for 2 min and incubated for 10 min in a cresyl violet bath. The next sections were rinsed twice with differentiation solution. They were then dehydrated through a sequence of ethanol baths (100, 90, 80, 70, and 50%). The sections were finally cleaned in xylene for 5 min and mounted with coverslips using Permount (Fair Lawn, NJ, USA).
Evaluation of BBB permeability
A 2% solution of Evans blue in normal saline (0.2 ml/mouse) was injected intravenously 24 h after tMCAO. The stain was allowed to circulate for 1 h. In sham-operated animals, intravenous injection of Evans blue was also performed. The stain was allowed to circulate for 1 h. The brains were collected and dehydrated using 30% sucrose. Immunohistochemistry was performed on 30 µm sections. Fluorescence signals were detected using a Zeiss epifluorescence microscope at excitation/emission wavelengths of 630/657 nm.
Western blot analysis
Whole proteins were extracted from cells or tissues. The concentration was determined by BCA assay, and 30 μg of the protein was loaded on 10%~12% SDS-PAGE gel. Electrophoresis was performed for approximately 2 h at 80 V. Then, the protein was transferred to the PVDF membrane, and the primary antibody was incubated overnight at 4℃. Primary antibodies against Ngb were diluted to 1:1000 (RD181043050, BioVendor, Asheville, NC, USA). The secondary antibody was incubated for 1 h at room temperature. The ECL reagent was used to detect target proteins, while β-actin was used to normalize the experimental error.
Immunohistochemistry
The brains were perfused with 4% paraformaldehyde in PBS (pH 7.4) and frozen. Immunohistochemistry was performed on 30 µm sections using rabbit polyclonal anti-mouse Ngb (Sigma 1:100), mouse monoclonal anti-NeuN (Chemicon; 1:200), rat monoclonal anti-PDGFRβ (Abcam; 1:100), mouse monoclonal anti-αSMA (Abcam; 1:100), mouse monoclonal anti-glial fibrillary acidic protein (Sigma; 1:200), as primary antibodies, and Alexa Fluor 488-conjugated goat anti-rat IgG (Abcam; 1:500), Alexa Fluor 594-conjugated goat anti-mouse IgG (Invitrogen; 1:500), and Alexa Fluor 546-conjugated goat anti-rabbit IgG (Invitrogen; 1:500) as secondary antibodies. Controls included omitting primary or secondary antibodies. Fluorescence signals were detected using a Zeiss LSM 800 confocal laser scanning microscope at excitation/emission wavelengths of 495/519 (Alexa Fluor 488), 556/573 (Alexa Fluor 546), 590/617 (Alexa Fluor 594), and 358/461 (DAPI) nm. In order to quantify Ngb expressed around blood vessels, we measured Ngb signals within 2 mm of all blood vessels using 512×512-pixel figures by image J.
Fluorescence-activated cell sorting
The brain tissue was minced and digested with 5 ml collagenase/dispase (2 mg/ml) in HBSS for 30 min in a 37℃ water bath, triturated with a 14G needle 20 times, and filtered with 40 μm cell strainer. Percoll (22%) was used to remove debris and myelin. After centrifugation at 700 G for 10 min, the supernatant was discarded. The cell pellet was resuspended in staining buffer and stained with PE anti-CD13 (1:20) and anti-Ngb (1:50) antibodies. APC anti-rabbit IgG antibody was used as the secondary antibody to label the Ngb antibody. The labeled cells were detected using fluorescence-activated cell sorting (FACS) Canto (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Data are expressed as the mean±standard deviation (SD). Statistical analysis was performed using GraphPad Prism software (GraphPad, San Diego, CA, USA). Comparisons between two groups were performed by unpaired t-test. Comparisons among multiple groups were performed using two-way ANOVA with Dunnett’s multiple comparison test. Student’s t-test was used to compare the mean values of two independent groups for each variable. Statistical significance was set at *p<0.05, **p<0.01, and ***p<0.001.
RESULTS
Distribution Ngb in NVU
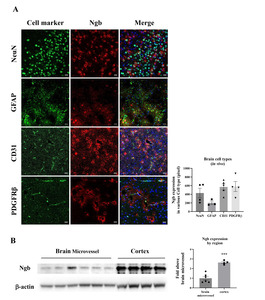
Ngb is the globin of neurons, and its distribution and role in neurons and glia have been studied [15,16]. However, the functions of Ngb in other brain cells, including the NVU, have not been studied. In this study, we found that Ngb is expressed not only in neurons and astrocytes, but also in other brain cells. IHC fluorescence staining of brain tissue showed that Ngb (red) was present in various cell types (green) constituting the BBB, including neurons, astrocytes, endothelial cells, and PDGFRβ-positive pericytes (Fig. 1A). Moreover, immunoblotting of the isolated cerebral blood vessels also showed the expression of Ngb, although it was 67% less than that in the cerebral cortex (Fig. 1B). It was confirmed that Ngb is distributed not only in neurons and astrocytes, but also in the NVU consisting of endothelial cells and pericytes in the normal brain. Next, we investigated the distribution and role of Ngb in cerebral ischemia.
Differential levels of Ngb in the core and penumbra after tMCAO in mice
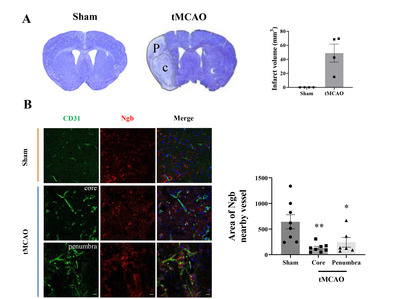
To observe how Ngb changes in brains with oxidative hypoxic damage, we used a mouse model of stroke with tMCAO. After stroke, cresyl violet was used to identify the infarct area, and the change in Ngb expression near the infarct area was analyzed (Fig. 2A). Ischemic injury due to stroke is comprised of the core and penumbra. The core is the area of severe ischemia and necrosis of neuronal glial cells, while the penumbra is a rim of mild to moderately ischemic tissue that may remain viable for several hours. The core and penumbra were classified based on cresyl violet staining (Fig. 2A). To observe the correlation between the levels and distributions of Ngb in the cranial nerve vascular system, Ngb (red) was double stained along with the vascular marker CD31 (green) (Fig. 2B). In the sham group, the expression of Ngb was near the blood vessel marker, CD31. In the tMCAO group, Ngb was decreased in the core of the cerebral infarct compared with the sham group, but levels of Ngb increased in the penumbra (peripheral tissue of the core). Moreover, after stroke, the Ngb around blood vessels was detached from the NVU in the core and was observed to be increased in neurons [17]. In the penumbra of tMCAO, although less than that in the sham group, there was a larger amount of Ngb around the blood vessel compared with that in the core (Fig. 2B). Quantitative analysis showed that Ngb existed around the blood vessel, and it was reduced to 20% and 37% in the core and penumbra, respectively, compared with the sham group (Fig. 2C).
Different distribution patterns of Ngb levels depending on the subtypes of pericytes (PDGFRβ or αSMA) after stroke
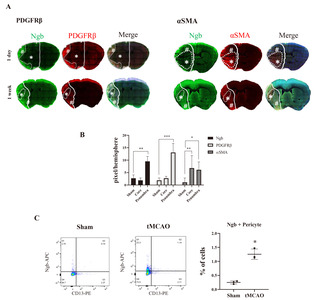
Since Ngb was detected in and around endothelial cells, we analyzed the distribution of Ngb in various types of pericytes. Pericytes are multifunctional mural cells around endothelial cells. There are many different types of pericytes. Platelet-derived growth factor receptor β (PDGFRβ) [18], alanyl aminopeptidase (CD13) [19], proteoglycan neuron-glial antigen 2 (NG2) [20], and desmin [21] are markers often used to identify various types of pericytes, with the α smooth muscle actin (αSMA) being used to define a contractile sub-class of pericytes [22]. The expression of all these markers changes during growth and development and may be up- or downregulated under pathological conditions [9]. We mainly observed pericytes labeled with PDGFRβ or αSMA, which is a contractile sub-class of pericytes. In PDGFRβ, its expression pattern was similar to that of Ngb, which was decreased in the core and increased in the penumbra 24 h and 1 week after tMCAO (Fig. 3A). On the other hand, the expression of αSMA increased in the core 24 h after tMCAO, which persisted in the core of the infarct by one week (Fig. 3A). These results indicate that the distribution of Ngb differs depending on the subtype of pericytes (Fig. 3B). Our results showed that the pattern of Ngb expression was similar to that of PDGFRβ-labeled pericytes (Fig. 3B), which implied an association of Ngb with PDGFR signal in the NVU after cerebral ischemia. To confirm the amount of pericytes expressing Ngb after tMCAO in the whole brain, the sham or ischemic brains were minced and homogenized, respectively. These were analyzed by immunostaining with Ngb (APC) and pericytes (CD13-PE) using FACS. The Ngb-expressing cells increased by 5-fold from the ipsilateral side of the ischemic brain, compared with the sham brain (Fig. 3C). In the whole brain, the pericytes expressing Ngb were 0.25% in the sham group and 1.25% in the ischemic brain. After stroke, increased Ngb and PDGFRβ pericytes were confirmed in the ischemic brain using IHC and FACS. Therefore, it is necessary to investigate the function of Ngb in the NVU during stroke.
Reduced BBB leakage with Ngb-positive pericytes in the penumbra after tMCAO
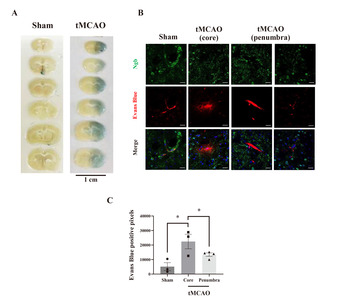
In hypoxic states or stroke, some pericytes migrate away from the endothelial cells [3]. This movement of pericytes leads to BBB breakdown and tight junction disintegration [23]. Our results have shown that Ngb is expressed in pericytes around the blood vessels of the sham group but is reduced in the ischemic core after stroke. This indicates the possibility that the existence of Ngb in pericytes plays an important role in maintaining the function of the BBB after stroke. To test whether the distribution of Ngb was involved in BBB integrity, we measured the extent of BBB leakage in the core or penumbra, along with the amount of Ngb-positive pericytes after stroke.
Evans blue (EB) was injected 24 h after tMCAO, leakage was measured by fluorescence excitation/emission wavelength of 630/657 nm, and we found increased BBB leakage on the ipsilateral side of the brain of mice receiving tMCAO (Fig. 4A). Simultaneously, the level of Ngb was analyzed by IHC staining after EB injection. In the sham group, EB dye existed only inside vessels and Ngb was distributed around the blood vessel, which showed no BBB leakage (Fig. 4B). However, in the tMCAO group, EB leaked into the brain tissue around the blood vessels, and the Ngb level was reduced. In particular, there was a lot of leakage in the core of the infarct, and Ngb disappeared around the blood vessel (Fig. 4B). Quantifying leaked EB by Image J showed that the core and penumbra leaked 4.3 and 2.6 times more, respectively, in the tMCAO group than in the sham group (Fig. 4C). These results showed that Ngb exists in the pericytes around blood vessels in the healthy brain and functions to maintain the BBB barrier. However, in oxidative cerebral ischemia conditions, it was found that Ngb was decreased in the pericytes around the NVU, which is highly affected by BBB leakage.
DISCUSSION
This study investigated the distribution and role of Ngb in pericytes after tMCAO in mice. We found that Ngb was increased in the penumbra, which was colocalized with PDGFR-positive pericytes. Interestingly, the presence of Ngb-positive pericytes reduced BBB leakage in the penumbra after cerebral ischemia. Ischemic stroke is primarily a consequence of cerebrovascular disease; therefore, manipulating the pericytes of the NVU is important for developing therapeutic strategies for stroke. In particular, pericytes are early responders to brain hypoxia, and they change from a quiescent flat shape into an amoeboid morphology after ischemia. In addition, pericytes may differentiate into other cell types and thus may be important for central nervous system renewal. They can differentiate into the basic components of the NVU, including vascular cells and glial cells [24].
First, we found that Ngb is expressed not only in neurons and astrocytes but also in pericytes and endothelial cells of the brain, implying a potential role of Ngb in NVU. Pericytes are multifunctional mural cells that surround endothelial cells. Controlling the crosstalk between endothelial cells and pericytes in the NVU is essential for the regeneration of neural cells and governs microvessel stability under tMCAO [25].
Ischemia induces PDGF-B expression by endothelial cells and PDGFRβ expression by pericytes. Activation of PDGFRβ by PDGF-B induces the phosphorylation of Akt in pericytes, resulting in an anti-apoptotic response and promotion of pericyte proliferation [8]. On the other hand, αSMA is a contractile pericyte that constricts capillaries during and after ischemia, which causes the no-reflow phenomenon after stroke [26].
We found that Ngb colocalized with PDGFRβ-positive pericytes in the penumbra after tMCAO; however, it was decreased in the core region. On the other hand, αSMA expression increased in the core rather than in the penumbra 24 h after stroke and was not colocalized with Ngb. Our results indicate that the distribution and role of Ngb are different in the various subtypes of pericytes depending on the severity of damage, such as in the ischemic core or penumbra. In other words, in the penumbra, the interaction of Ngb with PDGFRβ signaling might contribute to protection against oxidative stress, but not with the αSMA signal. Recently, it was reported that αSMA-positive type-2 pericytes were related to BBB disruption in brain disorders [27]. Selective regulation of pericyte subtype associated with Ngb is necessary to generate novel therapeutic approaches for protecting the BBB against oxidative brain damage.
Our results showed that stroke increased the expression of PDGFRβ signal in the penumbra, and Ngb may support the survival of pericytes, at least in part through its interaction with PDGFR β signals. Modulation of the Ngb level in pericytes might be promising for accomplishing NVU reconstruction during CNS regeneration and repair after stroke.
Pericytes detach from the basal lamina as early as 1 h after ischemic stroke [28], and detachment could lead to disruption of BBB integrity [29]. Therefore, the presence of pericytes in the NVU protects against BBB leakage. For example, reduced pericyte coverage in microvessels, which is a hallmark of diabetic retinopathy, induces decreased BBB stability and subsequent pathological changes [30].
We found increased BBB leakage on the ipsilateral core where Ngb-negative PDGFR was absent in the ischemic brain at 24 h after tMCAO. However, Ngb-positive PDGFRβ pericytes were present around the cerebral blood vessels in the penumbra after tMCAO and reduced BBB leakage. These findings suggest that the existence of Ngb-positive PDGFRβ pericytes in NVU is beneficial for maintaining BBB integrity after stroke.
Direct transplantation of pericytes, medication treatment to preserve pericyte function, and other possible therapeutic strategies are all possible new solutions for ischemic stroke. Several medicines have been reported to preserve pericyte function after ischemic brain injury. For example, the widely used antiplatelet drug cilostazol has been shown to prevent the detachment of pericytes and astrocyte endfeet from microvessels in spontaneous hypertensive rats with spontaneous cerebral infarcts. In reperfusion injury, oxidative stress and free radicals serve as the main toxic factors to pericytes. Edaravone, a free radical scavenger, promotes pericyte proliferation and increases the pericyte coverage of endothelial cells, which attenuates BBB destruction during reperfusion injury [31].
Pericytes are much more abundant than supportive cells in endothelial cells. These are important functional components of the BBB and NVU. They display significant alterations during ischemia and reperfusion and actively participate in brain injury, cell preservation, and brain repair [32]. Therefore, it is necessary to identify a factor that can increase the number of pericytes. Our findings suggest that Ngb is an endogenous neuroprotective factor that preserves the existence of pericytes. Strategies to modulate pericyte response via Ngb after ischemia and reperfusion may provide new therapies for ischemic stroke. We identified a few natural compounds that upregulated Ngb expression using a cell-based screening system [33]. In future studies, we may use these Ngb-upregulating compounds, such as flavonoids, to treat stroke animals to induce Ngb-pericyte-mediated BBB protection.
Our results indicate that Ngb plays a role in PDGFRβ signaling to reduce the number of cells in the penumbra after stroke. This implies that Ngb may be involved in the protective mechanism of pericytes during reperfusion injury after stroke. Further research is necessary to elucidate the mechanism of action of Ngb in pericytes. To demonstrate the Ngb-mediated neuroprotective mechanism in the pericytes of NVU, BrdU experiments are needed in Ngb transgenic mice to provide direct evidence of Ngb-mediated preservation of pericytes in the brain over time. Moreover, the cellular function of Ngb in pericytes is needed to investigate the mechanism of Ngb in subcellular locations, either in the cytoplasm or the nucleus. Until now, the subcellular location of Ngb has been known to be in the mitochondria and cytoplasm, which inhibit apoptosis by interacting with cytochrome C. In this study, we observed that Ngb was found in the nucleus when pericytes were detached from blood vessels after ischemic stroke. The role of Ngb in the cytoplasm and nucleus is expected to be different, and further studies are needed.
In the present study, we demonstrated for the first time that Ngb existed not only in neurons and astrocytes, but also in pericytes that form the NVU in the brain. In cerebral ischemia, a pattern appears in which Ngb is detached from the NVU and is very low in the ischemic core. However, the distribution of Ngb in PDGFRβ-positive pericytes was high in the penumbra of the infarct and resulted in reduced BBB leakage. Future studies may use Ngb-upregulating compounds to treat animals in the acute phase of stroke to preserve pericyte-mediated BBB integrity. These investigations may spur the development of therapeutic strategies targeting the protection of the BBB for neurological disorders such as stroke and vascular dementia.
ACKNOWLEDGEMENTS
This research was supported by grants from the Bio & Medical Technology Development and National R&D Program through the National Research Foundation of Korea (NRF-2018R1D1A1B07048027, NRF-2022R1A5A2021216), which are funded by the Ministry of Science and ICT.
Figures
References
- Sweeney MD, Ayyadurai S, Zlokovic BV (2016) Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19:771-783
- McConnell HL, Kersch CN, Woltjer RL, Neuwelt EA (2017) The translational significance of the neurovascular unit. J Biol Chem 292:762-770
- Liu S, Agalliu D, Yu C, Fisher M (2012) The role of pericytes in blood-brain barrier function and stroke. Curr Pharm Des 18:3653-3662
- Hu X, De Silva TM, Chen J, Faraci FM (2017) Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res 120:449-471
- Tanriover G, Sozen B, Seker A, Kilic T, Gunel M, Demir N (2013) Ultrastructural analysis of vascular features in cerebral cavernous malformations. Clin Neurol Neurosurg 115:438-444
- Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA (2000) Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res 60:55-69
- Cheng J, Korte N, Nortley R, Sethi H, Tang Y, Attwell D (2018) Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol 136:507-523
- Arimura K, Ago T, Kamouchi M, Nakamura K, Ishitsuka K, Kuroda J, Sugimori H, Ooboshi H, Sasaki T, Kitazono T (2012) PDGF receptor β signaling in pericytes following ischemic brain injury. Curr Neurovasc Res 9:1-9
- Armulik A, Genové G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193-215
- Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA (2006) Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A 103:17944-17948
- Burmester T, Hankeln T (2009) What is the function of neuroglobin?. J Exp Biol 212(Pt 10):1423-1428
- Brittain T, Skommer J, Raychaudhuri S, Birch N (2010) An antiapoptotic neuroprotective role for neuroglobin. Int J Mol Sci 11:2306-2321
- Yu Z, Cheng C, Liu Y, Liu N, Lo EH, Wang X (2019) Author correction: Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis 10:212
- Zhu LL, Zhao T, Li HS, Zhao H, Wu LY, Ding AS, Fan WH, Fan M (2005) Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res 1055:1-6
- Chen XQ, Qin LY, Zhang CG, Yang LT, Gao Z, Liu S, Lau LT, Fung YW, Greenberg DA, Yu AC (2005) Presence of neuroglobin in cultured astrocytes. Glia 50:182-186
- Burmester T, Weich B, Reinhardt S, Hankeln T (2000) A vertebrate globin expressed in the brain. Nature 407:520-523
- Kim SD, Kim M, Wu HH, Jin BK, Jeon MS, Song YS (2021) Prunus cerasoides extract and its component compounds upregulate neuronal neuroglobin levels, mediate antioxidant effects, and ameliorate functional losses in the mouse model of cerebral ischemia. Antioxidants (Basel) 11:99
- Lindahl P, Johansson BR, Levéen P, Betsholtz C (1997) Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277:242-245
- Kunz J, Krause D, Kremer M, Dermietzel R (1994) The 140-kDa protein of blood-brain barrier-associated pericytes is identical to aminopeptidase N. J Neurochem 62:2375-2386
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB (2001) NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222:218-227
- Nehls V, Denzer K, Drenckhahn D (1992) Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res 270:469-474
- Nehls V, Drenckhahn D (1993) The versatility of microvascular pericytes: from mesenchyme to smooth muscle?. Histochemistry 99:1-12
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV (2013) Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol 23:303-310
- Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, Kawahara M, Taguchi A, Matsuyama T (2015) Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 33:1962-1974
- Cao L, Zhou Y, Chen M, Li L, Zhang W (2021) Pericytes for therapeutic approaches to ischemic stroke. Front Neurosci 15:629297
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508:55-60
- Bohannon DG, Okhravi HR, Kim J, Kuroda MJ, Didier ES, Kim WK (2020) A subtype of cerebrovascular pericytes is associated with blood-brain barrier disruption that develops during normal aging and simian immunodeficiency virus infection. Neurobiol Aging 96:128-136
- Duz B, Oztas E, Erginay T, Erdogan E, Gonul E (2007) The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology 55:279-284
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C (2010) Pericytes regulate the blood-brain barrier. Nature 468:557-561
- Willard AL, Herman IM (2012) Vascular complications and diabetes: current therapies and future challenges. J Ophthalmol 2012:209538
- Deguchi K, Liu N, Liu W, Omote Y, Kono S, Yunoki T, Deguchi S, Yamashita T, Ikeda Y, Abe K (2014) Pericyte protection by edaravone after tissue plasminogen activator treatment in rat cerebral ischemia. J Neurosci Res 92:1509-1519
- Cai W, Liu H, Zhao J, Chen LY, Chen J, Lu Z, Hu X (2017) Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res 8:107-121
- Liu N, Yu Z, Gao X, Song YS, Yuan J, Xun Y, Wang T, Yan F, Yuan S, Zhang J, Xiang S, Lo EH, Wang X (2016) Establishment of cell-based neuroglobin promoter reporter assay for neuroprotective compounds screening. CNS Neurol Disord Drug Targets 15:629-639





