Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2022; 31(5): 332-342
Published online October 31, 2022
https://doi.org/10.5607/en22033
© The Korean Society for Brain and Neural Sciences
Visuosocial Preference Memory, but Not Avoidance Memory, Requires PLCγ1 in the CA2 Hippocampus
Sunpil Kim1,2, Jeongyeon Kim3, Yongmin Mason Park2,4, Pann-Ghill Suh5 and C. Justin Lee1,2,4*
1KU-KIST Graduate School of Converging Science and Technology, Korea University, Seoul 02841, 2Center for Cognition and Sociality, Institute for Basic Science (IBS), Daejeon 34126, 3Emotion, Cognition and Behavior Research Group, Korea Brain Research Institute (KBRI), Daegu 41062, 4IBS School, University of Science and Technology (UST), Daejeon 34113, 5Korea Brain Research Institute (KBRI), Daegu 41062, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-42-878-9150, FAX: 82-42-878-9151
e-mail: cjl@ibs.re.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Visuosocial memory is defined as stored visual information containing social context. Primates have a powerful ability to associate visuosocial memory with episodic memory. However, the existence of visuosocial memory in mice remains unclear. Here, we design a novel vision-specific social memory test using a portrait picture or mirrored self-image and demonstrate that mice can distinguish conspecific from other species by forming a visuosocial memory. Because CA2 hippocampus has been reported as a critical brain region for social memory, we develop CA2-specific blockade of memory formation through deletion of phospholipase C gamma 1 (PLCγ1), which is a key molecule in the brain-derived neurotrophic factor (BDNF) signaling pathway. Interestingly, these mice have intact sociability but impaired social memory in three chamber test and five-trial social memory test, which is highly dependent on visual information. Finally, PLCγ1 deletion in CA2 impairs visuosocial preference memory, but not avoidance memory, whereas non-social object recognition is intact. Our study proposes that mice have visuosocial memory, just as primates and humans.
Graphical Abstract
Keywords: Visuosocial memory, CA2 hippocampus, Phospholipase C gamma 1, Social behavior
INTRODUCTION
Social animals can quickly and accurately discriminate conspecifics using various external sensory information such as visual, auditory, olfactory, and tactile cues [1, 2]. Humans are extremely efficient in integrating social information into episodic memory. In contrast, rodents, the most widely used animals for social behavior, have been thought to have poor vision compared to humans. Thus, most of the sociality-related studies with rodents focus on olfactory social cues, whereas very few studies have focused on “visuosocial memory”. Visuosocial memory can be defined as one kind of social memory which contains visual information associated with social context. One potential brain region to store visuosocial memory is the hippocampus because; 1) Hippocampus has been considered as a key brain region to associate different types of information such as space, object, sound cues, and context into episodic memory [3-5], and 2) Recently the CA2 hippocampus was suggested as the critical brain region for social memory in mouse [6, 7]. Interestingly, receptors of oxytocin and vasopressin which are well-known for their function in social cognition are highly and specifically expressed in hippocampal CA2 [8, 9]. In addition, CA2 has been recently established as a distinct area with specific molecular markers such as Amigo2 and RGS14 with unique electrophysiological properties [10, 11]. CA2 has been revealed to play an essential role in social memory and exhibit long-term potentiation (LTP) at the entorhinal cortical input to CA2 synapses in mice [6, 12, 13]. However, up to date, no study has demonstrated the existence of visuosocial memory in rodents and the role of CA2 in this particular memory.
Memory can be formed by associating various types of stimuli with valence. For example, mice can associate a novel place with negative valences such as foot shock-induced fear [14] or empathic fear [15]. In contrast, mice can be conditioned to prefer a specific place by the treatment with DAMGO, a μ-opioid receptor (MOR) agonist [16]. Therefore, if visuosocial memory exists in mice, mice should associate a visuosocial stimulus with electric foot shock or DAMGO to establish visuosocial avoidance memory or visuosocial preference memory, respectively. During this association process, specific signaling pathways or molecules in CA2 must be involved.
Considerable lines of accumulating evidence demonstrate that brain-derived neurotrophic factor (BDNF) is required for synaptic plasticity and hippocampus-dependent memory [17, 18]. In animal studies, BDNF signaling in the hippocampus and mesolimbic circuit plays an important role in antidepressant action to relieve chronic social stress [19, 20]. Phospholipase C gamma 1 (PLCγ1) is known to be direct downstream signaling molecule of BDNF and its high-affinity receptor, tyrosine receptor kinase B (TrkB) [21]. Also, a previous report suggested that PLCγ1 is necessary for TrkB-mediated long-term potentiation in CA1 hippocampus [22]. However, little is known about whether PLCγ1-mediated BDNF signaling in CA2 is linked with social cognition and memory. In this study, we investigated the role of CA2 PLCγ1 in associating an episodic experience with visuosocial stimulus by utilizing a novel CA2-specific deletion of PLCγ1 mouse model, RGS14-cre×PLCγ1f/f.
MATERIALS AND METHODS
Animals
PLCγ1f/f mice were developed and genotyped as previously described [23]. They were maintained as heterozygotes on the C57BL/6J background and crossed to obtain homozygote mutants. RGS14-cre mouse (036535-UCD, MMRRC) was crossed with PLCγ1f/f mouse (PLCγ1f/f×RGS14-cre) for CA2-specific knockout of PLCγ1. To validate the CA2-specificity of RGS14-cre, tdTomatof/f mouse (007914, Jackson Laboratory) was crossed with RGS14-cre mouse (RGS14-cre×tdTomatof/f). To validate PLCγ1 expression level, triple transgenic mice were used (PLCγ1f/f×RGS14-cre×tdTomatof/f). Wildtype C57BL/6J and C57BL/6N mice were used for visuosocial memory test. All experiments were performed with 8 to 16-week-old male mice. Mice were kept on a 12 hr light-dark cycle in a specific-pathogen-free facility with controlled temperature and humidity and had free access to food and water. All experimental procedures, animal care, and handling were performed according to the directives of the Animal Care and Use Committee and the institutional guidelines of IBS (Daejeon, Korea).
Behavioral tests
Mice were group-housed two to five in each cage and had free access to food and water. They were kept on a 12 hr (8:00 to 20:00) light-dark cycle in the mouse facility with tightly maintained temperature (18 to 22°C) and humidity (40 to 60%). All tests were performed at a similar time in the light cycle (14:00 to 20:00). Mice were gently handled daily for 3 days before the first day of experiments. Mice were given at least 1 hr to habituate after moving to the behavioral room before experiments. All behavior experiments were performed under 10 to 20 lux light intensity except the ‘Darkroom shock’ experiment (complete dark, 0 lux).
Visuosocial memory test
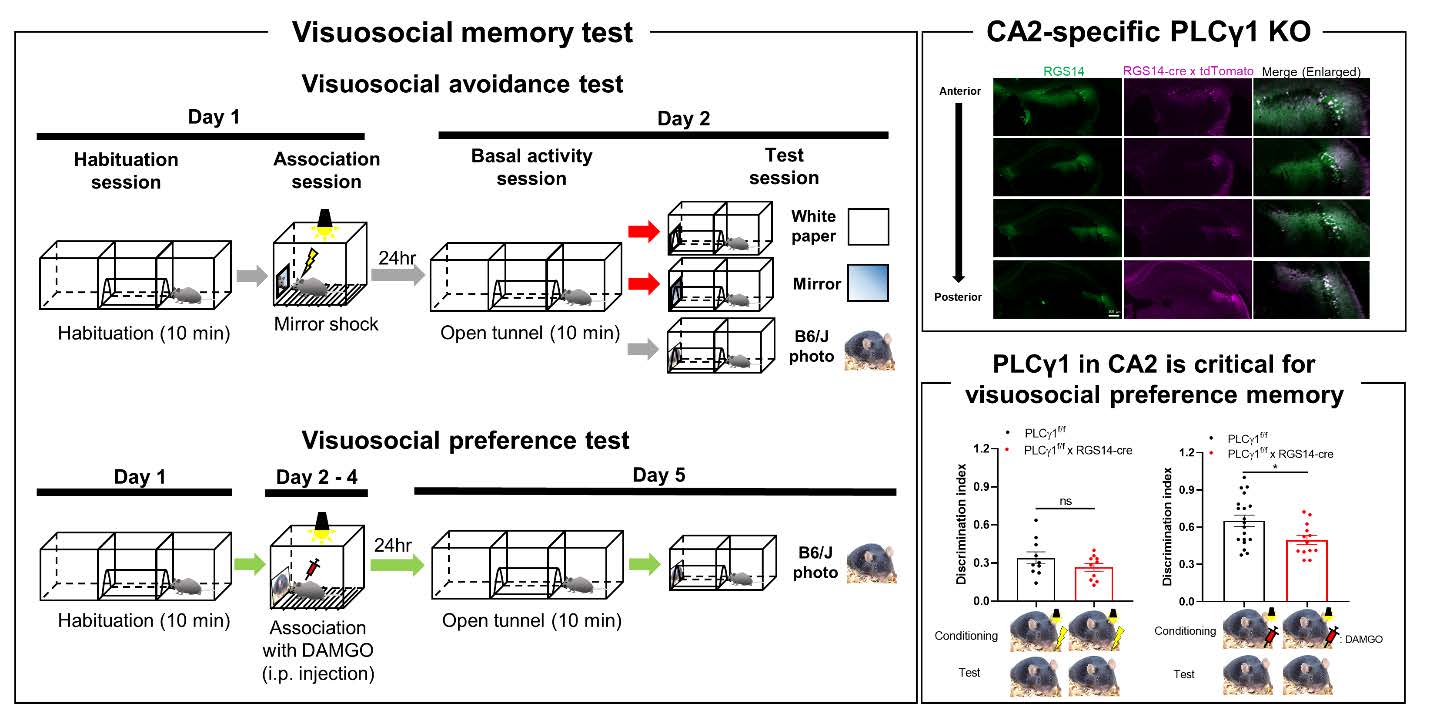
The overall experimental scheme of visuosocial avoidance and preference memory tests is described in Fig 1. For visuosocial avoidance test, one day before the experiment, the subject was allowed to explore the 2-chamber arena connected by a tunnel in the middle for 10 min to acclimate the apparatus (40 cm length×20 cm width×20 cm height for each chamber). Mice passed a tunnel at least one time in habituation session went to next session. On Day 1, one more habituation session (10 min) was done. Right after habituation, mice were placed into a fear conditioning chamber (H10-11M-TC, Coulbourn, see Fig. 1D). In this chamber, a visuosocial cue (mirror, pictures of C57BL/6, Balb/c, or chipmunk, see Fig. 1E) was attached at one side of the wall. The subject received the electric foot shock (0.3 mA, 2 sec) 2 times when the mouse interacted with the cue (head toward the cue). The subject returned to the home cage 1 min after the last shock. On Day 2, mice were freely moved in the arena same as on Day 1 for 10 min (Open tunnel session). After that, the cue was installed at the end of the tunnel and the number of entering the tunnel was recorded (Test session). For a control cue, white paper was used. To normalize the activity during the test session by basal activity, we used the discrimination index for comparison (Entering frequency in test session / Entering frequency in open tunnel session). We counted entering numbers only when a whole mouse body entered the tunnel. For the ‘No shock’ control experiment, mice were placed into the fear conditioning chamber with a mirror for 5 min without any electric shock. For the ‘Darkroom shock’ control experiment, there was no light in the chamber, and mouse behavior was recorded by the infrared camera. For visuosocial preference test, instead of foot shock, DAMGO (1 mg/kg, i.p., 1171, Tocris) was injected right before transferring into the fear conditioning chamber and let mice stay for 30 min for 3 consecutive days (see Fig. 1B). We found that there is no significantly different entering frequency between before and after stimulation (foot shock or DAMGO) in both control and PLCγ1 cKO groups by comparing open tunnel session of Day 1 with Day 2 (foot shock) or Day 5 (DAMGO).
Immunohistochemistry
Mice were deeply anesthetized with 2% avertin (i.p.) and perfused with 0.9% saline followed by ice-cold 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS). Excised brains were post-fixed overnight in 4% PFA at 4°C and immersed in 30% sucrose for over 24 hr for cryoprotection. Brain slices (30 μm, coronal) containing the hippocampus were obtained. Sections were incubated for 1 hr in a blocking solution (0.3% Triton–X 100, 2% goat serum, and 2% donkey serum in 0.1M PBS) and then immunostained with a primary antibody (anti-RGS14, 1:50 dilution; 73-170; NeuroMab) in a blocking solution at 4°C on a shaker overnight. After washing in 0.1M PBS 3 times, sections were incubated with secondary antibody (anti-mouse 488, 1:500 dilution; 715-545-150; Jackson ImmunoResearch) for 1 hr and then washed with PBS 3 times. Finally, sections were mounted with a fluorescent mounting medium (S3023, Dako). All slice images were obtained by slide scanner (Axio scan.Z1, Zeiss).
Brain cell isolation
RGS14-cre×tdTomatof/f and PLCγ1×RGS14-cre×tdTomatof/f mice were deeply anesthetized with 3% isoflurane and brains from these mice were quickly removed from the skull and submerged in ice-cold 0.1 M PBS. For brain cell isolation, the adult brain dissociation kit (130-107-677, Miltenyi Biotec) was used for brain cell isolation following the manufacturer’s instructions. After dissociation, to collect tdTomato-positive cells specifically, fluorescence-activated cell sorting (FACS) experiment was performed using MoFlo Astrios Cell Sorter (Beckman Coulter). FACS-sorted tdTomato-positive cells were used for qRT-PCR and western blot to detect PLCγ1 mRNA and protein level, respectively. We used 2 mice brains to make one sample. For qRT-PCR and western blot, we used 2 samples for each condition with duplicate or triplicate.
Quantitative real-time PCR (qRT-PCR)
Using FACS-sorted cells, RNA extraction (Qiagen RNeasy mini kit, 74104) and cDNA synthesis (SuperScriptTM III First-Strand Synthesis System, Invitrogen) were performed following the manufacturer’s instructions. After cDNA synthesis, qRT-PCR (QuantStudio 1, Applied Biosystems) was performed in triplicates in a total volume of 20 μl containing 10 pM primers (forward and reverse), 4 ul cDNA, and 5 ul Power SYBR Green Master Mix (4367659, Applied Biosystems). The following sequences of primers were used for qRT-PCR.
PLCγ1 forward: 5’-CCG GCC AGA TCA ATC ACA CT-3’; PLCγ1 reverse: CCG GAG CCA CCT CTC AAT TT.
GAPDH forward: 5’- ACC CAG AAG ACT GTG GAT GG -3’; GAPDH reverse: 5’-CAC ATT GGG GGT AGG AAC AC-3’.
Western blot
For cell lysis, FACS-sorted cells were mixed with a lysis buffer containing 50mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 10 mM NaF, and protease and phosphatase inhibitor cocktail. The total protein concentration was determined with the BCA protein assay. Equal amounts of samples were loaded into 8% acrylamide gel and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE). After separation, gel was electrophoretically transferred to polyvinylidene fluoride (PVDF, Millipore) membrane by the gel transfer device (iBlot 2, Invitrogen). The membranes went through a blocking step using 5% skimmed milk in TBS-T buffer (Tris-buffered saline containing 0.05% Tween 20) for 1 hr. The membranes were incubated with primary antibody overnight at 4°C. After washing in TBS-T buffer, the membranes were incubated with horseradish peroxidase-labeled secondary antibody for 1 hr at room temperature. Protein bands were visualized by ECL reagents (GE Healthcare). Monoclonal anti-PLCγ1 antibody was generated as previously described [24]. For loading control, anti-β-actin (691001, MP Biomedicals) antibody was used.
Three chamber test
The test was performed in three sessions within a three-chambered open arena (40 cm length×20 cm width×20 cm height for each chamber). In the habituation session (Day 1), the subject freely explored the apparatus for 10 min. In the sociability session (Day 2), the subject encountered a stranger mouse within a metal cup and an empty metal cup for 10 min. In the social novelty session (Day 3), the subject encountered the familiar one as well as a novel mouse in another metal cup in the social novelty session for 10 min. The duration for exploring each cup was analyzed.
Five-trial social memory test
This test was run as described previously with a minor modification [25]. In brief, animals were housed individually for 7~10 days before testing. On testing, a female C57BL/6 stimulus mouse was introduced into the home cage of each subject for 1 min and then returned to an individual holding cage. Four trials (1~4 trials) were repeated with 10 min inter-trial intervals and the same stimulus to the resident in all four trials. In a fifth dishabituation trial, a novel stimulus female mouse was introduced. The time spent in the olfactory investigation for each trial was recorded and analyzed.
Novel object recognition test
Test was conducted in an open field arena (40 cm length×40 cm width×40 cm height) with two different kinds of objects (A, B). Initially there was no preference in both objects. During habituation, mouse was allowed to explore an empty arena for 10 min. 24 hr after habituation, the subject was exposed to the familiar arena with two identical objects (A, A’) placed at an equal distance for 10 min (Acquisition). 1 hr later the subject explored the open field in the presence of the familiar object and a novel object (A, B) to test recognition memory for 10 min (Retention). The time spent exploring each object and the discrimination index was analyzed.
Statistical analyses
All statistical analyses were performed using Prism 9 (GraphPad). Differences between two different groups were analyzed with a two-tailed Student’s t-test. For comparison of multiple groups, one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test, or two-way ANOVA with Sidak’s multiple comparison test was assessed. Animals were randomly and evenly allocated to each group for all experiments. All data were presented as mean±SEM. Asterisks indicate a significant difference as follow: ns, not significant, p>0.05; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
RESULTS
To directly test whether mouse has an ability to form visuosocial memories, we designed a novel visuosocial memory test (Fig. 1, see detail in materials and methods session), in which mice learn to associate mirrored self-image or mouse-photo associated with electric shock-induced fear (visuosocial avoidance) or DAMGO-induced positive valence (visuosocial preference).
After pairing mirrored self-image or mouse-photo with electric shock, mice entered significantly less into the mirror- or photo-installed tunnel, compared to no-mirror control (white paper), no-shock control, or dark room condition (Fig. 2A), suggesting that mouse is capable of forming visuosocial avoidance memories based on vision-specific fear-associated social cues. Unexpectedly, the C57BL/6-photo-shock paired mice showed a significant avoidance not only to C57BL/6-photo but also to Balb/c-photo and vice versa (Fig. 2B). This avoidance to the conspecific (Balb/c-photo) was not due to avoidance to the Balb/c-photo itself because the no-shock control showed no avoidance (Fig. 2B). Because C57BL/6 and Balb/c are conspecific, we hypothesized that mice can distinguish conspecific from heterospecific. To test this hypothesis, we used a photo of a chipmunk, a heterospecific animal. Surprisingly, mice did not avoid C57BL/6-photo when they received electric shock with chipmunk-photo or vice versa (Fig. 2C). In contrast, mice showed avoidance to chipmunk-photo when they received electric shock with chipmunk-photo (Fig. 2C), indicating that mice can distinguish conspecific from heterospecific and form visuosocial memories. Furthermore, mice can successfully associate not only visuosocial cues but also a neural cue (food pellet-photo) with electric shock-induced fear (Fig. 2D). Lastly, we test whether mice can associate positive valence with C57BL/6 photo using DAMGO (Fig. 2E). Interestingly, opposite to avoidance test, mice treated with DAMGO increased discrimination index, indicating that mice can associate positive valence with visuosocial cue (Fig. 2E). Taken together, using our novel visuosocial memory test, we discovered that mice can associate visuosocial cues with negative valence (fear) or positive valence (DAMGO).
To investigate the involvement of BDNF signaling in social memory in CA2, we utilized a previously reported CA2-specific cre-expressing mouse, RGS14-cre [26]. We validated cre expression in CA2 hippocampus using RGS14-cre crossed with tdTomatof/f mice (Fig. 3A). Then, to remove PLCγ1 selectively in CA2 pyramidal neurons, we additionally crossed PLCγ1f/f mice to make triple transgenic mice (PLCγ1f/f×RGS14-cre×tdTomatof/f). Using qRT-PCR and western blot, we found a significant reduction of PLCγ1 mRNA and protein expression in FACS-sorted cre-expressing cells (Fig. 3B~E). Taken together, we successfully developed CA2-specific PLCγ1 KO using PLCγ1f/f×RGS14-cre mice.
To examine whether PLCγ1 in CA2 is involved in social behavior, we performed three-chamber test with PLCγ1f/f (Control) and PLCγ1f/f×RGS14-cre (PLCγ1 cKO) mice to assess sociability and social novelty (Fig. 4A~F). We firstly found that there was no difference in locomotor activity between control and PLCγ1 cKO (Data not shown). We also found that there was no impairment of sociability in both groups (Fig. 4B, C), but impaired social novelty in PLCγ1 cKO group (Fig. 4E, F). This finding was consistent with the previous report of normal sociability but impaired social novelty in mice with CA2 pyramidal neuron inactivation by overexpression of tetanus-neurotoxin [6].
We performed another well-known social memory test, the five-trial social memory test (Fig. 4G), in which the process of familiarization is assessed. We found that PLCγ1 cKO group showed a significantly impaired familiarization at the fourth trial, compared to the control group (Fig. 4H), suggesting that familiarization requires PLCγ1 in CA2. The impaired familiarization in PLCγ1 cKO group suggested that PLCγ1 is involved in a subset of social cues. To test if visuosocial cue is required for familiarization, we performed the same five-trial social memory test in the dark condition (Dark control) with naïve C57BL/6 mice. Surprisingly, interaction time in the entire trial was significantly reduced in the dark control condition (Fig. 4H), suggesting that the visuosocial cues are required for social interaction but not for familiarization. Deletion of PLCγ1 in CA2 did not alter the ability to recognize non-social objects (Fig. 4I, J), suggesting that CA2 might be specific for visuosocial memory. Taken together, we concluded that PLCγ1 in CA2 is critical for both social novelty memory and familiarization which requires visuosocial information.
Finally, to evaluate which valence is assoicated with visuosocial cue by PLCγ1 in CA2, we tested both visuosocial avoidance memory and visuosocial preference memory with control and PLCγ1 cKO mice. We found that PLCγ1 cKO mice showed no significant difference in visuosocial avoidance memory with C57BL/6 photo (Fig. 5A), as well as with a neutral cue (Fig. 5B). On the other hand, PLCγ1 cKO mice showed significantly decreased visuosocial preference memory (Fig. 5C), whereas neutral cue (food pellet photo)-induced preference memory was intact (Fig. 5D), indicating that PLCγ1 in CA2 is crucial for visuosocial preference memory. Also, these data suggested that mice recognize C57BL/6 photo as a social stimulus which is fundamentally different from a neutral object such as a food pellet. We additionally confirmed that these behavioral results were not affected by foot shock or DAMGO (Fig. 5E). Based on these results, we concluded that PLCγ1 in CA2 is critical for visuosocial preference memory, but not avoidance memory.
DISCUSSION
In this study, we have developed a novel animal model and behavioral test using visuosocial cue-fear (Foot shock) or positive valence (DAMGO) association to examine the neural network and molecular mechanisms of visuosocial memory. Our results propose that CA2 PLCγ1, which is one of the key components of BDNF signaling, is critically involved in visuosocial preference memory, but not avoidance memory. DAMGO is known to activate MOR in CA1 hippocampal astrocyte to release glutamate which activates presynaptic mGluR1 to enhance synaptic plasticity and cause conditioned place preference [16]. Therefore, it is highly possible that visuosocial preference memory is formed through the similar mechanism by CA2 astrocytic MOR. Also, during social memory tests (three chamber test and five-trial social memory test) and visuosocial preference memory test, BDNF is released to CA2 neurons to induce PLCγ1-mediated synaptic potentiation. These interesting hypotheses should be examined in the future.
In contrast to visuosocial preference memory, we found that visuosocial avoidance memory is unaltered in KO. This raises a possibility that visuosocial avoidance memory might be encoded in other hippocampal areas. One possible candidate can be ventral CA1, which has been suggested as a critical brain region for social memory [27] and fear memory encoding [28]. This interesting possibility should be investigated in the future.
There is one potential caveat of using RGS14-cre mouse. Even though RGS14-cre can target CA2 specifically in hippocampus, we observed cre expression in other brain areas such as piriform area and lateral part of cerebellum. Therefore, we cannot completely rule out the possible role of PLCγ1 in the formation of visuosocial preference memory in those brain areas.
The molecular, genetic, and behavioral tools that we have developed will be useful in understanding the social memory processing in social animals including human, who is very well known for having a high capacity for visuosocial memories through the ability to recognize conspecifics by visual cues. Moreover, it would be the first step to addressing a critical question of how human remembers conspecific’s face and associated memories. Finally, we propose that the BDNF-TrkB-PLCγ1 axis in CA2 can be a novel therapeutic target for impaired facial information processing and related diseases such as prosopagnosia, dementia, and autism.
ACKNOWLEDGEMENTS
This work is supported by the Institute for Basic Science (IBS), Center for Cognition and Sociality (IBS-R001-D2) funded by the Ministry of Science to C. J. L and KBRI basic research program through Korea Brain Research Institute (22-BR-03-04) and the National Research Foundation of Korea (NRF) grant (2019R1F1A1061867) funded by Ministry of Science and ICT to J. K.
Figures





References
- Schellinck HM, Price SR, Wong MJ (2008) Using ethologically relevant tasks to study olfactory discrimination in rodents. In: Chemical signals in vertebrates 11 (Hurst JL, Beynon RJ, Roberts SC, Wyatt TD edsBuccafusco JJ edHurst JL, Beynon RJ, Roberts SC, Wyatt TD eds), pp 71-80. Springer, New York, NY
- Haxby JV, Hoffman EA, Gobbini MI (2002) Human neural systems for face recognition and social communication. Biol Psychiatry 51:59-67
- Burgess N, Maguire EA, O'Keefe J (2002) The human hippocampus and spatial and episodic memory. Neuron 35:625-641
- Buzsáki G, Moser EI (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci 16:130-138
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S (2007) Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317:94-99
- Hitti FL, Siegelbaum SA (2014) The hippocampal CA2 region is essential for social memory. Nature 508:88-92
- Lopez-Rojas J, de Solis CA, Leroy F, Kandel ER, Siegelbaum SA (2022) A direct lateral entorhinal cortex to hippocampal CA2 circuit conveys social information required for social memory. Neuron 110:1559-1572.e4
- Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, Froemke RC (2016) A distributed network for social cognition enriched for oxytocin receptors. J Neurosci 36:2517-2535
- Pagani JH, Zhao M, Cui Z, Avram SK, Caruana DA, Dudek SM, Young WS (2015) Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol Psychiatry 20:490-499
- Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, Wickersham IR, Tonegawa S (2014) Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci 17:269-279
- Laeremans A, Nys J, Luyten W, D'Hooge R, Paulussen M, Arckens L (2013) AMIGO2 mRNA expression in hippocampal CA2 and CA3a. Brain Struct Funct 218:123-130
- Dudek SM, Alexander GM, Farris S (2016) Rediscovering area CA2: unique properties and functions. Nat Rev Neurosci 17:89-102
- Chevaleyre V, Siegelbaum SA (2010) Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron 66:560-572
- Curzon P, Rustay NR, Browman KE (2009) Cued and contextual fear conditioning for rodents. In: Methods of behavior analysis in neuroscience (Hurst JL, Beynon RJ, Roberts SC, Wyatt TD edsBuccafusco JJ edHurst JL, Beynon RJ, Roberts SC, Wyatt TD edsBuccafusco JJ ed). 2nd ed, pp 19-37. CRC Press/Taylor & Francis, Boca Raton, FL
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS (2010) Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci 13:482-488
- Nam MH, Han KS, Lee J, Won W, Koh W, Bae JY, Woo J, Kim J, Kwong E, Choi TY, Chun H, Lee SE, Kim SB, Park KD, Choi SY, Bae YC, Lee CJ (2019) Activation of astrocytic μ-opioid receptor causes conditioned place preference. Cell Rep 28:1154-1166.e5
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257-269
- Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R (1999) Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24:401-414
- Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Männistö PT, Castrén E (2007) Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology 32:2152-2162
- Wook Koo J, Labonté B, Engmann O, Calipari ES, Juarez B, Lorsch Z, Walsh JJ, Friedman AK, Yorgason JT, Han MH, Nestler EJ (2016) Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol Psychiatry 80:469-478
- Minichiello L (2009) TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10:850-860
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M (2002) Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 36:121-137
- Yang YR, Jung JH, Kim SJ, Hamada K, Suzuki A, Kim HJ, Lee JH, Kwon OB, Lee YK, Kim J, Kim EK, Jang HJ, Kang DS, Choi JS, Lee CJ, Marshall J, Koh HY, Kim CJ, Seok H, Kim SH, Choi JH, Choi YB, Cocco L, Ryu SH, Kim JH, Suh PG (2017) Forebrain-specific ablation of phospholipase Cγ1 causes manic-like behavior. Mol Psychiatry 22:1473-1482
- Suh PG, Ryu SH, Choi WC, Lee KY, Rhee SG (1988) Monoclonal antibodies to three phospholipase C isozymes from bovine brain. J Biol Chem 263:14497-14504
- DeVito LM, Konigsberg R, Lykken C, Sauvage M, Young WS 3rd, Eichenbaum H (2009) Vasopressin 1b receptor knock-out impairs memory for temporal order. J Neurosci 29:2676-2683
- Jeong Y, Huh N, Lee J, Yun I, Lee JW, Lee I, Jung MW (2018) Role of the hippocampal CA1 region in incremental value learning. Sci Rep 8:9870
- Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S (2016) Ventral CA1 neurons store social memory. Science 353:1536-1541
- Kim WB, Cho JH (2020) Encoding of contextual fear memory in hippocampal-amygdala circuit. Nat Commun 11:1382





