Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2022; 31(6): 390-400
Published online December 31, 2022
https://doi.org/10.5607/en22034
© The Korean Society for Brain and Neural Sciences
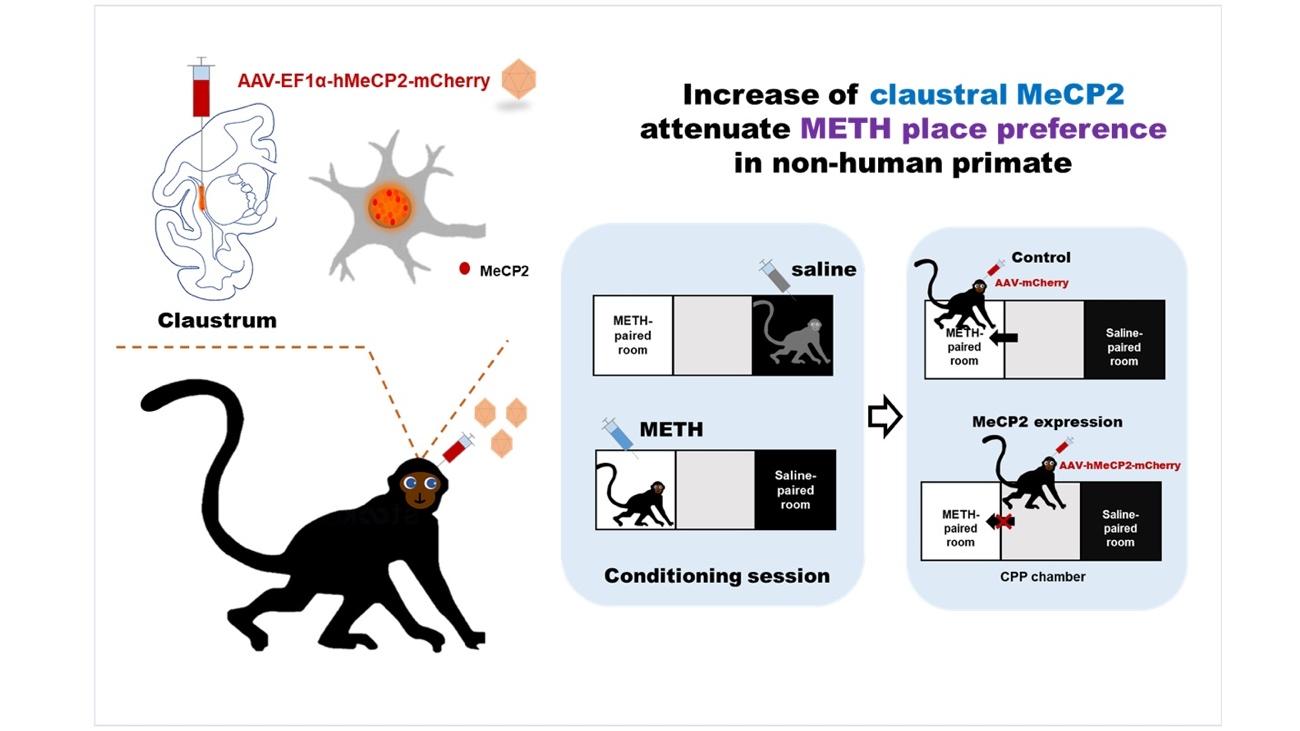
Claustral MeCP2 Regulates Methamphetamine-induced Conditioned Place Preference in Cynomolgus Monkey
Jinhee Bae1, Sujin Ahn1, Doo-Wan Cho2, Hyung-Sun Kim2, Su-Cheol Han2 and Heh-In Im1,3*
1Center for Brain Function, Brain Science Institute, Korea Institute of Science and Technology, Seoul 02792, 2Jeonbuk Branch Institute, Korea Institute of Toxicology, Jeongeup 56212, 3Division of Bio-Medical Science & Technology, KIST School, Korea University of Science and Technology, Seoul 02792, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-958-6961, FAX: 82-2-958-6937
e-mail: him@kist.re.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The claustrum, a brain nucleus located between the cortex and the striatum, has recently been highlighted in drug-related reward processing. Methyl CpG-binding protein-2 (MeCP2) is a transcriptional regulator that represses or activates the expression of the target gene and has been known to have an important role in the regulation of drug addiction in the dopaminergic reward system. The claustrum is an important region for regulating reward processing where most neurons receive dopamine input; additionally, in this region, MeCP2 is also abundantly expressed. Therefore, here, we hypothesized that MeCP2 would be involved in drug addiction control in the Claustrum as well and investigated how claustral MeCP2 regulates drug addiction. To better understand the function of human claustral MeCP2, we established a non-human primate model of methamphetamine (METH) - induced conditioned place preference (CPP). After a habituation of two days and conditioning of ten days, the CPP test was conducted for three days. Interestingly, we confirmed that virus-mediated overexpression of MECP2 in the claustrum showed a significant reduction of METH-induced CPP in the three consecutive days during the testing period. Moreover, they showed a decrease in visit scores (frequency for visit) for the METH-paired room compared to the control group although the scores were statistically marginal. Taken together, we suggest that the claustrum is an important brain region associated with drug addiction, in which MeCP2 may function as a mediator in regulating the response to addictive drugs.
Graphical Abstract
Keywords: Methyl-CpG-binding protein 2, Claustrum, Drug addiction, Methamphetamine, Cynomolgus monkey
INTRODUCTION
Methyl CpG-binding protein-2 (MeCP2) is a transcriptional regulator that represses or activates the expression of target genes by binding to methylated DNA [1, 2]. MeCP2 is abundantly expressed in the neurons of adult brains [3], and MeCP2 mutations cause neurobehavioral abnormalities that range from mild cognitive disabilities to neurodevelopmental disorders including autism and Rett syndrome [4, 5].
Drug addiction causes behavioral changes by altering the function of several decisive molecules implicated in dopaminergic pathways [6, 7]. In recent decades, the relationship between MeCP2 and drug addiction has been identified [6, 8-10]. The decrease of MeCP2 in the dorsal striatum attenuated cocaine consumption in the self-administration test of rodents [8], and the increase of MeCP2 in the NAc (nucleus accumbens) reduced the amphetamine-induced conditioned place preference [9]. These results suggest that MeCP2 has an important role in modulating the addiction response in the dopaminergic reward system.
The claustrum is a thin sheet of gray matter located in between the insula and striatum. Although it has been previously reported to be associated with attention, sleep, and consciousness [11-13], more recently, the claustrum has been highlighted as a pivotal area related to reward processing and drug addiction [14-19]. The claustrum receives dopaminergic innervation from midbrain dopamine centers such as the ventral tegmental area and substantia nigra, and more than half of the neurons are composed of neurons expressing dopamine receptors [18, 20, 21]. The study that explored the reward-related role of the claustrum showed that the inhibition of the claustrum activity reduces cocaine addiction-related responses in the conditioned place preference and sensitization tests [18]. Specifically, when rewards are presented, the claustrum helps to initiate attention and salience responses for the rewards and forms cue–reward associations [16, 17]. In the early stages of the reward and cue association, the neural activity of the claustrum is increased from the cue onset of signal initiation to the reward delivery, and this claustral activity helps to form associations between the reward and the correct cue. Furthermore, it has been reported that the inhibition of claustral neurons projecting to the medial prefrontal cortex attenuated methamphetamine-induced impulsive behavior [19]. Although the claustrum is one of the important brain regions in drug addiction, the effect of epigenetic modifications in the claustrum on addiction behavior remains poorly understood.
MeCP2 has been reported to regulate drug addiction within the dopamine pathway and is abundantly expressed in the Claustrum [22]. Therefore, we hypothesized that the claustral MeCP2 could be closely involved in the regulation of the addictive drug responsiveness and confirmed how MeCP2 in claustrum regulates drug addiction. To this end, we developed a primate model of the METH-induced conditioned place preference test and investigated whether the regulation of claustral MeCP2 affects METH preference. In addition, we sought to better understand the function of human claustral MeCP2 in drug addiction by using a primate model that is more physiologically similar to humans than rodents.
MATERIALS AND METHODS
Animals
Four adult-male cynomolgus monkeys were used in the present study, weighting in at 2.6~3.6 kg prior to their enrollment in this study. The monkeys were tagged and housed in individual cages for a year or more since their arrival at the Korea Institute of Toxicology (KIT). The monkeys were housed individually under standard conditions (a 12-hour light/dark cycle with the light on from 08:00 to 20:00 hours, humidity at 30~70% and temperature at 23±3°C) in the animal facility. Institutional Animal Care and Use Committee (IACUC) approval for the experimental procedures at the Korean Institute of Toxicology (Approval Number: KIT-1610-0367, protocol Number: B216062, 18 October 2016) was obtained before the start of the study. All animal care was performed following the guideline of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Virus preparation and surgery
Human full-length MeCP2 (486 amino acids) was cloned into pAAV-Ef1a-mCherry (control vector, Addgene, Plasmid #114470). Constructs were packaged in the AAV-DJ/8 Rep-Cap plasmid (Cell biolabs, #VPK-420-DJ-8). Monkeys were deeply anesthetized with about 8 mg/kg zolazepam hydrochloride (zoletil®50) and were bilaterally injected with 30 ul (2.5 ul/min) volumes into the claustrum of each hemisphere [coordinates from the bregma according to Szabo and Cowan [23]: +19 mm anteroposterior (AP), ±13.5 mm mediolateral (ML) and -16 mm dorsoventral (DV)]. After injection and dose delivery, a 26-gauge needle Hamilton syringe was left in the injection site for 10 min and was then slowly withdrawn.
Conditioned place preference test
Four weeks after the surgery, monkeys were subjected to the conditioned place preference test (CPP) (Fig. 1). During the habituation phase, the monkeys were individually guided into the center room where they were left to freely explore the three rooms for 50 minutes. Each room was allocated as either center, meth-paired, or saline-paired rooms based on the initial preference formed during the habituation phase. The meth-paired room was assigned based on where the monkeys spent relatively the least amount of time in each of the rooms. The room opposite to the meth-paired room was assigned as the saline-paired room. After the two-day habituation period, the monkeys were trained for CPP. During the conditioning phase, monkeys were either given METH (3 mg/ml/kg) or saline injections on an alternating injection scheme. The conditioning phase began with a saline injection. During the conditioning phase, the monkeys were given either METH or saline injection in their home cage. At 5 min post-injection, they were confined in the respective rooms for 50 min (days 4, 6, 8, 10 and 12 with METH and days 3, 5, 7, 9 and 11 with saline). After ten days of conditioning, a post-test was conducted, and the monkeys were tested for CPP. In the post-test phase, the monkeys were given access to freely explore the three rooms after entering the middle room without any METH or saline injections. The post-test was performed for 50 minutes for three consecutive days.
Apparatus
The CPP test was conducted in a CPP chamber suspended 48.5 cm from the floor. The apparatus consisted of three-square rooms connected laterally (Fig. 1). The size of each room was 60×70×78 cm (l×w×h). Each room was colored for high contrast (black, gray, or white), and the center room colored gray was designated as the start zone. The CPP chamber was kept at a constant temperature of 23℃ (±3℃) and illuminated at 300~700 lux.
Drug
Methamphetamine hydrochloride (3 mg/ml/kg) provided by the National Institute of Food and Drug Safety Evaluation (NIFSD, South Korea) was dissolved in saline (0.9%) for subcutaneous (SC) injection. A 0.9% saline solution was used as the vehicle control on alternating days with the METH injections.
Immunohistochemistry
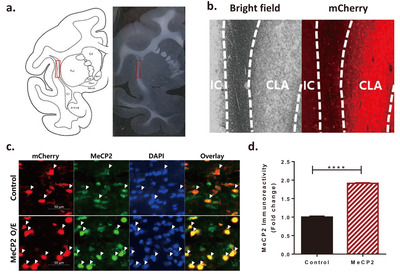
Monkeys were deeply anesthetized with 0.8 g/kg thiopental sodium one day after P3. Brains were harvested and immediately immersed in 4% ice-cold PFA and incubated at 4℃ on a shaker for 3 days (72 hours). Then, the brains were incubated in 10% sucrose at 4°C on a shaker until submersion and then sequentially incubated in 20% and 30% sucrose. Brains were coronally sectioned (40 μm) in a cryostat at -20℃ (Leica, Wetzlar, Germany). Sectioned brain slices were washed three times in 1X PBS followed by blocking in 5% normal goat serum and 0.3% Triton X-100 in PBS for 2 hours. The brain sections were incubated overnight at 4℃ in Rabbit anti-MeCP2 (1:250, 07-013, Millipore, MA, USA) and Chicken anti-mCherry (1:1,000, B205402, Abcam, Cambridge, U.K.). After washing three times, the sections were incubated in Alexa Fluor 488 goat anti-rabbit IgG (1:400, A-11008, Life Technologies, CA, USA) and Alexa Fluor 594 goat anti-chicken IgG (1:500, A-11042, Life Technologies, CA, USA). Finally, the sections were washed three times and then mounted onto coverslips using a mounting medium with DAPI solution (H-1500, Vector Laboratory, CA, USA). Confocal images were taken by using a Zeiss LSM800 Confocal Microscope (Oberkochen, Germany). MECP2 immunoreactivity was analyzed using the ImageJ software from the National Institutes of Health (MD, USA).
Measurement and data analysis
A camera was installed in front of the center room, and the video was recorded by a computer outside the room. The activity was measured using the Vigie Primates V2.5 software, and the thresholds were set as previously described by Liu et al. [24]. The statistical package PRIZM 6 was used for the data analysis. Analyses for the CPP were conducted by comparison of the test trials with the pre-CPP levels seen on the last habituation trial (H2) [25]. The preference scores were calculated as follows: [post-test: duration of visits in the METH-paired room/(duration of visits in the METH-paired room+duration in the saline-paired room)]–[habituation day2 (pre-test): duration of visits in the METH-paired room/(duration of visits in the METH-paired room+duration in the saline-paired room)]. Larger CPP scores represent a stronger preference for METH. The visit score (the frequency of visits to the METH-paired room) and the locomotion score (the distance moved in the METH-paired room) were also calculated using the aforementioned formula. Analyses for the behavioral sensitization effect induced by repeated addictive drug exposure were performed by comparing the distance moved during the METH and the saline conditioning trials in the final six days (C5-C10) for the control and MeCP2 groups.
All data measurements including preference, visit, and locomotion scores, and the distance traveled were compared using the two-way mixed ANOVA analysis. After ANOVA analysis, multiple comparisons were performed with Fisher’s LSD. The significance level for all tests was set to a p≤0.05.
RESULTS
To understand how the claustral MeCP2 has a critical role in METH addiction, human MeCP2 was expressed in the claustrum by bilateral microinjection of the AAV virus that included the expression vector for the MeCP2 protein. The similarity of amino acids between human and cynomolgus monkeys was 99.8% (all matched except 1 amino acid out of 486). For the control group, a control vector (AAV-Ef1a-mCherry) in which MeCP2 was not cloned was injected into the claustrum. The injection site was validated by mCherry (Fig. 2a, b), and the efficiency of AAV-MeCP2 was confirmed with immunohistochemistry (Fig. 2c, d). Neurons expressing AAV-MeCP2 showed a stronger intensity of MeCP2 than neurons without the virus expression (
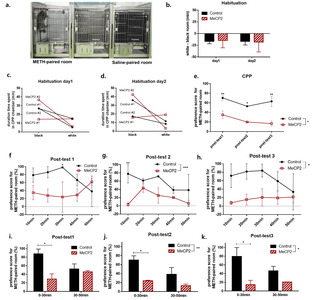
During the habituation phase, there was no difference in preference between both groups (Fig. 3b). Three monkeys preferred the black room to the white room, and one monkey had no preference for any specific room (Fig. 3c, d). Their non-preferred room (white room) was chosen as the drug-paired room and the preferred room (black room) as the saline-paired room [26].
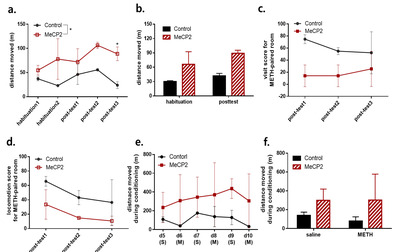
After ten days of conditioning, a post-test was conducted for three days. The expression of MeCP2 in the claustrum attenuated the preference for the 3 mg/kg METH-paired room in all post-test sessions (Fig. 3e). Although both groups showed more preference for the METH-paired room than for the saline-paired room, there were significant differences in the preference scores between the MeCP2 expression group and control group in all post-test sessions (virus group:
However, the CPP score for the MeCP2 expression group showed a tendency to be decreased in the third post-test session compared to the first post-test session, implying that the repeated exposure to the CPP room without METH injection attenuated the association between the METH and METH-paired room in the MeCP2 expression group (post-test day 1 versus day 3 in MeCP2 expression group:
Each post-test session was analyzed in 10 minute intervals, in which there was a significant difference in preference score between the virus groups in the second and third post-test sessions [virus group in post-test day1:
In further analysis, we confirmed that MeCP2 expression induced a higher level of distance moved compared with those in monkeys injected with the control virus [virus group:
Moreover, MeCP2 expression did not increase the visit score and the locomotion score for the METH-paired room (Fig. 4c, d). Although not statistically significant, additional analysis for the visit score in each post-test session revealed that the MeCP2 expression group showed a tendency to have lower visit scores than the control group on the first post-test sessions (post-test day1:
DISCUSSION
In this study, we confirmed that MeCP2 is an important factor in regulating the response to addictive drug in the claustrum. The increase of MeCP2 in the claustrum decreased the METH preference in cynomolgus monkeys. In all 3-day post-test sessions of the CPP, an increase of MeCP2 in the claustrum diminished the preference for the METH-paired room compared to the control. Additionally, although there was no statistical significance, the visiting frequency (visit score) to the METH-paired room was lower in the group with increased claustral MeCP2.
The function of the claustrum in drug addiction still is not fully understood. Recently, a small number of studies have reported that the claustrum may regulate reward processing and drug addiction [14, 18, 19]. Terem et al. have reported that the inhibition of claustrum neurons receiving dopaminergic inputs from ventral tegmental area and substantia nigra reduces cocaine sensitization and cocaine CPP [18]. In addition, optogenetic activation of frontal-projecting claustral neurons without the injection of addictive drugs during the CPP conditioning session induced real-time CPP, which indicates claustrum involvement in the reward system. Our study supports that the claustrum has an important role in controlling drug addiction responses. Moreover, the increase of MeCP2 in the claustrum seems to be involved in reducing the preference for METH and weakening the association with METH and the METH-paired room. In our non-human primate model, the CPP was strongly maintained in the control group even 3 days after conditioning. This result may indicate that once the preference for an addictive drug is formed, more time is required for the preference for the addictive drug to be extinguished. Indeed, previous studies have reported that the morphine-induced CPP was still maintained even 75 months after forming an association between morphine and a morphine-paired room [26, 28]. In this, the role of MeCP2 in relieving dependence on addictive drugs has biological significance because we found that an increase in MeCP2 in the claustrum lowered the preference for METH.
One thing to consider is that the effect of increasing MeCP2 on addictive drug preference is similar to that reported by Terem et al., that is, decreased addictive drug preference after inhibiting the activation of claustral neurons. It is possible that the increase of MeCP2 in the claustrum is related to the inactivation of the claustral neuronal population through various molecular pathways. Indeed, several studies have reported that MECP2 influences neuronal activation [9, 29, 30]. For instance, in hippocampal neurons of MeCP2 knockout mice, the MeCP2 knockout induced a decrease in the frequency of spontaneous excitatory synaptic transmissions (mEPSCs) compared to the wild type control [29]. In another study, the spine densities of GABAergic synapses in NAc were not enhanced in AMPH-treated mutant mice bearing a hypo-morphic mutation in
Another significance of our study is the development of a non-human primate model of METH CPP. So far, the METH-induced CPP test has not been performed in the non-human primate model. We first constructed the non-human primate model for the METH-induced CPP test. For this, we referred to other METH CPP rodent models and psychostimulant non-human primate CPP models reported in previous studies [26, 28, 31, 32]. The conditioning time was determined to be 50 minutes based on the plasma concentration peak found around 50 to 60 minutes in response to treatment with a METH dose of 1 and 5 mg/kg (SC) in rodents [31]. We also administered a METH dose of 3 mg/kg (SC) in the conditioning phase, in which METH induced CPP was confirmed in previous reports [33]. We found that the CPP was sufficiently formed at a METH dosage of 3 mg/kg with 50 minutes of 5 conditioning periods per day for 10 days in the non-human primate model. Additionally, we performed post-tests for 3 consecutive days to confirm the changes in the CPP level over time [26, 28]. In most of the studies that included the CPP test, the measurement time of the post-test was set within 15-30 minutes [26, 34]. We set the measurement time of the post-test to 50 minutes, which is longer than previously reported, to observe the changes in preference for the METH-paired room over time. As a result, the CPP did not decrease in both groups during the initial 30 minutes, and differences were not observed between the groups after 30 minutes, suggesting that setting the CPP test time to 30 minutes is sufficient [26, 32].
In this study, the expression of MeCP2 increased the locomotion compared with the control group. However, despite the increase in locomotion, the locomotion score for the METH-paired room in the MeCP2 expression group showed no difference compared to the control group. These results imply that the increase in locomotion may not be induced by addictive drugs. Instead, the increase in locomotion by increasing MeCP2 may be associated with the connectivity of claustrum with regions related to motor function such as the motor cortex [35], striatum [36, 37], and substantia nigra [18]. In addition, the increase in locomotion induced by the claustral MeCP2 elevation may be considered to be associated with various psychopathological symptoms in addition to the change in motor function. For example, the claustrum receives inputs from the basolateral amygdala and has been reported to be interconnected with the orbitofrontal cortex, an area associated with anxiety [38, 39], which implies claustrum involvement in emotional processing [37]. Existing animal studies have reported a negative correlation between the level of anxiety and the locomotion [40, 41]; thus, the enhanced locomotion induced by the increase in claustral MeCP2 may suggest a decrease in the level of anxiety in our study. However, further studies are needed as we did not verify whether claustral MeCP2 affects the regulation of anxiety levels.
Additionally, our data showed that in both the control and MeCP2 groups, saline and METH injections did not induce a difference in locomotion during the conditioning phase (Fig. 4e, f). Whereas, previous studies have shown that repeated psychostimulant exposure elicits a hyper-locomotor response in rodent models [42, 43]. Our results are similar to the reports utilizing non-human primate models that do not show changes in locomotion with CPP conditioning or repeated drug exposure [25-27], indicating that there may be variations in the addictive drug response due to interspecies differences in mesolimbic dopaminergic systems. For example, hyper-locomotion after repeated exposure to addictive drugs has been reported to be related to the dopaminergic system in the striatum of rodent models [44, 45]. Furthermore, although an increase in drug dose improved sensitization and led to an increase in the release of dopamine (DA) from the striatum of rodents [46], this effect was not observed in the monkey model [47, 48]. Moreover, in an human imaging study using PET scan, subjects who were repeatedly exposed to addictive drugs showed a decrease or no increase in addictive drug-induced DA release compared to the healthy control group [47, 49]. Therefore, the interspecies differences in striatal dopaminergic function may have induced various locomotion responses. In fact, studies using human subjects measure parameters of sensitization by tracking the changes in the levels of energy, mood, and verbalization along with eye blinking [50, 51]; in non-human primate studies, parameters such as verifying that the animal has visual confirmation of the environmental conditions or that it tracks non-existent objects have been used to measure sensitization [47].
In this study, we confirmed that claustral MeCP2 is an important factor in regulating addictive behavior. We also showed the effects gene expression regulation in the claustrum may have on the preference for addictive drugs. Moreover, this is the first study that investigates the role of MeCP2 on drug addiction in a non-human primate model. Although there have been studies that utilized rodent models to identify the role of MeCP2 in addiction, it is unclear whether these MeCP2 functions hold translative power in human drug addicts. Therefore, there is a need to identify the function of MeCP2 in drug addiction in a non-human primate model to improve its clinical application. In this study, we confirmed the role of MeCP2 in drug addiction by using a non-human primate model that expresses MeCP2 with nearly the same amino acid sequences found in human MeCP2 and by transducing human MeCP2 expression in the primate claustrum. Addictive drugs like METH causes a variety of molecular changes in various parts of the brain known to be altered by the administration of the drug [6, 52]. Thus, identifying molecules associated to the alteration of MeCP2 within the claustrum will increase the understanding of the neurological mechanisms for drug addiction and increase the likelihood of clinical applications.
ACKNOWLEDGEMENTS
We would like to thank Sangjoon Lee and Tae Kyoo Kim for revising the manuscript and assisting in the critical review of this paper.
FUNDING AND DISCLOSURE
This research was supported by National Research Council of Science & Technology (NST) grant by the Korean government (MSIP) (No. 2020R1A2C200461012) and the intramural grant (2E31700) from the Korea Institute of Science and Technology (KIST) to H. -I. Im. The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
J. Bae., S. Ahn., S. C. Han. and H. -I. Im. designed the experiment. D. W. Cho. and H. S. Kim. conducted viral injection into the monkey brain. J. Bae. and S. Ahn. performed the experiments and analyzed the data. J. Bae., S. Ahn. and H. -I. Im. wrote the manuscript. All authors reviewed the manuscript.
Figures
References
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393:386-389
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320:1224-1229
- Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY (2002) Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet 11:115-124
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23:185-188
- Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, Monteggia LM (2012) A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. J Neurosci 32:3109-3117
- Nestler EJ (2004) Molecular mechanisms of drug addiction. Neuropharmacology 47 Suppl 1:24-32
- Camí J, Farré M (2003) Drug addiction. N Engl J Med 349:975-986
- Im HI, Hollander JA, Bali P, Kenny PJ (2010) MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13:1120-1127
- Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE (2010) MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci 13:1128-1136
- Deng JV, Wan Y, Wang X, Cohen S, Wetsel WC, Greenberg ME, Kenny PJ, Calakos N, West AE (2014) MeCP2 phosphorylation limits psychostimulant-induced behavioral and neuronal plasticity. J Neurosci 34:4519-4527
- Renouard L, Billwiller F, Ogawa K, Clément O, Camargo N, Abdelkarim M, Gay N, Scoté-Blachon C, Touré R, Libourel PA, Ravassard P, Salvert D, Peyron C, Claustrat B, Léger L, Salin P, Malleret G, Fort P, Luppi PH (2015) The supramammillary nucleus and the claustrum activate the cortex during REM sleep. Sci Adv 1:e1400177
- Goll Y, Atlan G, Citri A (2015) Attention: the claustrum. Trends Neurosci 38:486-495
- Koubeissi MZ, Bartolomei F, Beltagy A, Picard F (2014) Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy Behav 37:32-35
- Graf M, Wong KLL, Augustine GJ (2020) Neuroscience: a role for the claustrum in drug reward. Curr Biol 30:R1038-R1040
- Smith JB, Lee AK, Jackson J (2020) The claustrum. Curr Biol 30:R1401-R1406
- Atlan G, Terem A, Peretz-Rivlin N, Sehrawat K, Gonzales BJ, Pozner G, Tasaka GI, Goll Y, Refaeli R, Zviran O, Lim BK, Groysman M, Goshen I, Mizrahi A, Nelken I, Citri A (2018) The claustrum supports resilience to distraction. Curr Biol 28:2752-2762.e7
- Smith JB, Watson GDR, Liang Z, Liu Y, Zhang N, Alloway KD (2019) A role for the claustrum in salience processing?. Front Neuroanat 13:64
- Terem A, Gonzales BJ, Peretz-Rivlin N, Ashwal-Fluss R, Bleistein N, Del Mar Reus-Garcia M, Mukherjee D, Groysman M, Citri A (2020) Claustral neurons projecting to frontal cortex mediate contextual association of reward. Curr Biol 30:3522-3532.e6
- Liu J, Wu R, Johnson B, Vu J, Bass C, Li JX (2019) The claustrum-prefrontal cortex pathway regulates impulsive-like behavior. J Neurosci 39:10071-10080
- Zingg B, Dong HW, Tao HW, Zhang LI (2018) Input-output organization of the mouse claustrum. J Comp Neurol 526:2428-2443
- Borroto-Escuela DO, Fuxe K (2020) On the G protein-coupled receptor neuromodulation of the claustrum. Neurochem Res 45:5-15
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168-176
- Szabo J, Cowan WM (1984) A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis). J Comp Neurol 222:265-300
- Liu N, Yue F, Tang WP, Chan P (2009) An objective measurement of locomotion behavior for hemiparkinsonian cynomolgus monkeys. J Neurosci Methods 183:188-194
- Borges AC, Duarte RB, Nogueira L, Barros M (2015) Temporal and dose-dependent differences in simultaneously-induced cocaine hypervigilance and conditioned-place-preference in marmoset monkeys. Drug Alcohol Depend 148:188-194
- Wang J, Wu X, Li C, Wei J, Jiang H, Liu C, Yu C, Carlson S, Hu X, Ma H, Duan W, Ma Y (2012) Effect of morphine on conditioned place preference in rhesus monkeys. Addict Biol 17:539-546
- Cagni P, Komorowski M, Melo GC, Lima T, Tomaz C, de Souza Silva MA, Huston JP, Barros M (2012) Repeated cocaine administration in marmoset monkeys induces hypervigilance-related behaviors, but no changes in locomotion and cortisol levels. Pharmacol Biochem Behav 103:279-283
- Yan T, Rizak JD, Wang J, Yang S, Ma Y, Hu X (2015) Severe dopaminergic neuron loss in rhesus monkey brain impairs morphine-induced conditioned place preference. Front Behav Neurosci 9:273
- Nelson ED, Kavalali ET, Monteggia LM (2006) MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol 16:710-716
- Fasolino M, Zhou Z (2017) The crucial role of DNA methylation and MeCP2 in neuronal function. Genes (Basel) 8:141
- Rambousek L, Kacer P, Syslova K, Bumba J, Bubenikova-Valesova V, Slamberova R (2014) Sex differences in methamphetamine pharmacokinetics in adult rats and its transfer to pups through the placental membrane and breast milk. Drug Alcohol Depend 139:138-144
- Wu X, Zhao N, Bai F, Li C, Liu C, Wei J, Zong W, Yang L, Ryabinin AE, Ma Y, Wang J (2016) Morphine-induced conditioned place preference in rhesus monkeys: resistance to inactivation of insula and extinction. Neurobiol Learn Mem 131:192-200
- Wakida N, Kiguchi N, Saika F, Nishiue H, Kobayashi Y, Kishioka S (2014) CC-chemokine ligand 2 facilitates conditioned place preference to methamphetamine through the activation of dopamine systems. J Pharmacol Sci 125:68-73
- Ning T, Gong X, Xie L, Ma B (2017) Gut microbiota analysis in rats with methamphetamine-induced conditioned place preference. Front Microbiol 8:1620
- Wang Q, Ng L, Harris JA, Feng D, Li Y, Royall JJ, Oh SW, Bernard A, Sunkin SM, Koch C, Zeng H (2017) Organization of the connections between claustrum and cortex in the mouse. J Comp Neurol 525:1317-1346
- Borra E, Luppino G, Gerbella M, Rozzi S, Rockland KS (2020) Projections to the putamen from neurons located in the white matter and the claustrum in the macaque. J Comp Neurol 528:453-467
- Benarroch EE (2021) What is the role of the claustrum in cortical function and neurologic disease?. Neurology 96:110-113
- Terburg D, Morgan BE, Montoya ER, Hooge IT, Thornton HB, Hariri AR, Panksepp J, Stein DJ, van Honk J (2012) Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry 2:e115
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH (2010) Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci 30:7023-7027
- Ranjbar H, Radahmadi M, Reisi P, Alaei H (2017) Effects of electrical lesion of basolateral amygdala nucleus on rat anxiety-like behaviour under acute, sub-chronic, and chronic stresses. Clin Exp Pharmacol Physiol 44:470-479
- Mesfin M, Asres K, Shibeshi W (2014) Evaluation of anxiolytic activity of the essential oil of the aerial part of Foeniculum vulgare Miller in mice. BMC Complement Altern Med 14:310
- Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99-120
- Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, Hope BT (2008) Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci 27:202-212
- Frey K, Kilbourn M, Robinson T (1997) Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharmaco 334:273-279
- Gou H, Wen D, Ma C, Li M, Li Y, Zhang W, Liu L, Cong B (2015) Protective effects of cholecystokinin-8 on methamphetamine-induced behavioral changes and dopaminergic neurodegeneration in mice. Behav Brain Res 283:87-96
- Liu Y, Roberts DC, Morgan D (2005) Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci 22:195-200
- Bradberry CW (2007) Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology (Berl) 191:705-717
- Bradberry CW, Rubino SR (2006) Dopaminergic responses to self-administered cocaine in Rhesus monkeys do not sensitize following high cumulative intake. Eur J Neurosci 23:2773-2778
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M (2007) Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 164:622-629
- Strakowski SM, Sax KW (1998) Progressive behavioral response to repeated d-amphetamine challenge: further evidence for sensitization in humans. Biol Psychiatry 44:1171-1177
- Narendran R, Martinez D (2008) Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse 62:851-869
- Chao J, Nestler EJ (2004) Molecular neurobiology of drug addiction. Annu Rev Med 55:113-132





