Articles
Article Tools
Supplementary
Stats or Metrics
Article
Original Article
Exp Neurobiol 2022; 31(6): 419-430
Published online December 31, 2022
https://doi.org/10.5607/en22006
© The Korean Society for Brain and Neural Sciences
Enhancing the Effect of Placental Extract on the Regeneration of Crush Injured Facial Nerve
Gyeong Min Lim1†, Gwang-Won Cho1,2†, Chitra Devi Ganesan2, Ji Hyun Choi3, Mary Jasmin Ang4, Changjong Moon4* and Chul Ho Jang5*
1BK21 FOUR Education Research Group for Age-Associated Disorder Control Technology, Department of Integrative Biological Science, Chosun University, Gwangju 61452, 2Department of Biology, College of Natural Science, Chosun University, Gwangju 61452, 3Department of Obstetrics and Gynecology, Chosun University School of Medicine, Gwangju 61452, 4Department of Veterinary Anatomy, College of Veterinary Medicine and BK21 FOUR Program, Chonnam National University, Gwangju 61186, 5Department of Otolaryngology, Chonnam National University Medical School, Gwangju 61469, Korea
Correspondence to: *To whom correspondence should be addressed.
Changjong Moon, TEL: 82-62-530-2838
e-mail: moonc@chonnam.ac.kr
Chul Ho Jang, TEL: 82-62-220-6774
e-mail: jchsavio@gmail.com
†These authors contributed equally to this article.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
There is a scarcity of experimental studies on peripheral nerve regeneration using placental extract (PE). This study aimed to investigate the effects of topical PE application on recovery after crush injury to the rat facial nerve using functional, electrophysiological, and morphological evaluations. The viability of the RSC96 Schwann cells treated with PE (0.5~4 mg/ml) increased significantly. Immunoblot test revealed that PE application enhanced the migration of RSC96 cells. Quantitative polymerase chain reaction demonstrated that PE increased the expression of neurotropic genes. The recovery from vibrissa fibrillation in the PE-treated group was superior to that in the control group. The threshold of action potential was also significantly lower in the PE group. Histopathological examination showed that crushed facial nerves treated with PE exhibited larger axons. The surrounding myelin sheaths were more distinct and thicker in the PE-treated group. Hence, PE may be considered a topical therapeutic agent for treating traumatic facial nerve paralysis.
Graphical Abstract
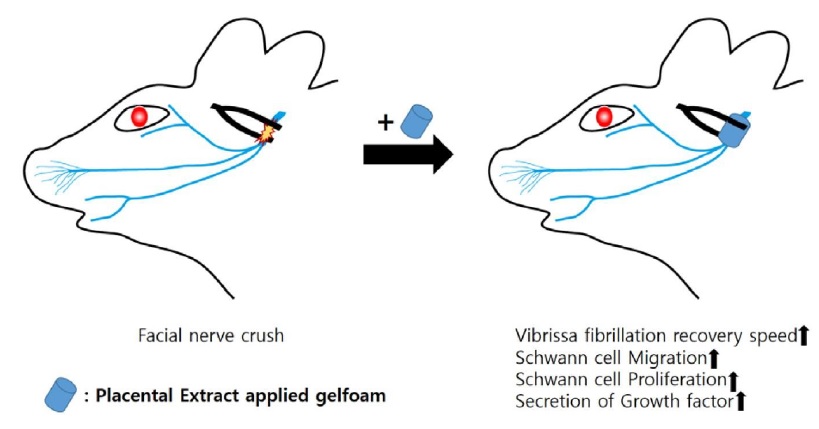
Keywords: Placental extract, Facial nerve, Crush injury, Regeneration
INTRODUCTION
Facial nerve palsy refers to paralysis of all structures innervated by the facial nerve, which inhibits facial expression. Trauma accounts for 10~23% of the etiology of facial nerve paralysis [1]. Importantly, facial nerve palsy has a significant impact on patients’ quality of life. Facial nerve trauma is responsible for the majority of the incidence of traumatic facial nerve paralysis. It is commonly caused by road traffic accidents, acute compression, or iatrogenic injury. Most temporal bone fractures are associated with intracranial injuries, and 10% occur in combination with cervical spine injuries [2].
Unlike the central nerves, the peripheral nerves undergo functional regeneration after injury by regrowing toward the injured area. Pharmacological treatment can help improve neurological function following peripheral nerve damage, especially crush injuries. To date, attempts have been made to regenerate the facial nerve after paralysis induced by crush injury in animal experiments using bioactive molecules known as growth factors, such as the basic fibroblast growth factor [3, 4], insulin growth factor [5], transforming growth factor-beta [6], and nerve growth factor [7].
Human placental extract (PE) is rich in biomolecules, cytokines, and growth factors such as epidermal growth factor, fibroblast growth factor, insulin growth factor, transforming growth factor, vascular endothelial growth factor, hepatocyte growth factor, and granulocyte-macrophage colony-stimulating factor, which potentiate regeneration, wound healing, cellular proliferation, and anti-inflammatory, antioxidant, and immunomodulatory effects (Supplementary Table) [8-20].
Experimental studies on peripheral nerve regeneration using PE are scarce, and only one study has explored its role in peripheral nerve recovery. However, it relied solely on histological evaluation, and no electrophysiological studies have been conducted [21].
Topical growth factor delivery is a promising procedure that can localize therapeutics to the facial nerve in the event of traumatic paralysis. To date, only a few experimental studies on traumatic facial nerve paralysis using topical single growth factors, such as basic fibroblast GF [22], insulin growth factor [5], brain-derived neurotrophic factor [23], neuregulin-1 [24], and glial growth factor [25], are available.
Gelfoam can be used for drug or gene delivery in sponge or gelatin hydrogel form [26]. According to Hom et al. [27], the effects of sustained release using gelfoam or poloxamer with endothelial cell growth factor did not show significant differences.
We hypothesized that multiple native growth factors present in PE would be considerably more effective than a single recombinant growth factor. To date, no study has investigated the effect of topical PE on the rat facial nerve following crush injury. The present study aimed to investigate the effects of topical PE application on recovery after crush injury to the rat facial nerve using functional, electrophysiological, and morphological evaluations.
MATERIALS AND METHODS
In vitro study
PE preparation
PE was prepared using a method described by Heo et al. [28], with some modifications. The human placental tissue was obtained from the Chosun University Hospital, Gwangju, South Korea. Human placental tissues were acquired with written consent and were approved by the Chosun University Hospital Institutional Review Board (CHOSUN 2022-10-024-002). The tissue was washed with ice-cold phosphate-buffered saline (PBS) to remove all traces of blood. Subsequently, the tissues were sectioned into small pieces and homogenized in cold PBS for approximately 10 min using a tissue homogenizer (Biospec Products Inc., OK, USA). The homogenates were centrifuged at 6,000 g for 20 min at 4℃. The supernatant was collected and lyophilized to yield powdered PE. PE (20 mg/ml) was then dissolved in 1× PBS and sterilized by filtering (0.22 µm), and subsequently used for further experiments.
Cell culture
RSC96 rat Schwann cells were cultured in high-glucose Dulbecco's modified Eagle’s medium (Gibco BRL, NY, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, ON, Canada) and 1% penicillin and streptomycin (Gibco, NY, USA) at 37℃ with 5% CO2 in a humidified atmosphere.
Cell viability assay
The proliferative effects of PE on RSC96 cells were evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (Sigma, MO, USA) according to the manufacturer’s instructions. Briefly, RSC96 cells were seeded in 96-well plates at a density of 1×104 cells/well. The next day, the cells were incubated with 0~4 mg/ml PE for 24 h, followed by analysis with the MTT assay.
Cell migration
RSC96 cells were seeded in 12-well plates at a density of 1×105 cells/well and were incubated at 37℃ for 24 h. The cells were treated with PE for 24 h at 37℃ after reaching 80% confluence. A sterile P200 pipette tip was used to scratch the cell monolayer and create a gap of approximately 1.0 mm. The area covered by migrating cells was measured using the Image J software (https://imagej.nih.gov).
Immunoblot analysis
Total proteins were extracted from RSC96 cells with 50 μl radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology, TX, USA) containing PMSF (Enzo Life Sciences, NY, USA) and an inhibitor cocktail (Thermo Fisher Scientific, MA, USA) for 30 min on ice and then centrifuged at 16,000 rpm for 20 min. The protein concentration was measured using a bovine serum albumin assay kit (Thermo Fisher Scientific, MA, USA). Protein samples were subsequently analyzed by immunoblot analysis with specific antibodies for proliferating cell nuclear antigen (PCNA) (1:500), extracellular signal-regulated kinase (ERK) (1:500), phosphorylated-ERK (p-ERK) (1:500), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:1,000) for 16 h at 4℃. The appropriate horseradish peroxidase-conjugated secondary antibodies were used for enhanced chemiluminescence detection.
RNA isolation and reverse transcription polymerase chain reaction
RNA was extracted from RSC96 Schwann cells using RNAiso Plus (TAKARA, Tokyo, Japan). cDNA was prepared using PrimeScript II 1st Strand cDNA Synthesis kits (TAKARA, Tokyo, Japan) with the supplied buffer (0.2 μg of random primers and 1 mM dNTPs). cDNA was amplified using the Power SYBR Green PCR Master Mix (Applied Biosystems, CA, USA). Reverse transcription polymerase chain reaction (RT-PCR) was performed using rat gene-specific primers for nerve growth factor (
In vivo study
Animals
All animal experiments were performed in accordance with the local ethics committee of the Chosun University (CIACUC2021-A0021). Experiments were conducted on 10 adult Sprague-Dawley rats weighing 250~300 g. Rats were anesthetized using isoflurane via inhalation.
Surgical procedures
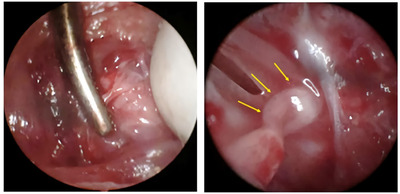
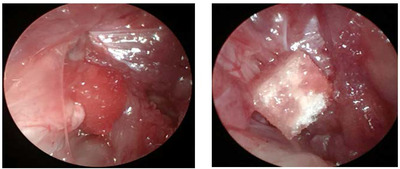
A post-auricular incision was made on the left side. The main trunk of the facial nerve was exposed under a surgical microscope (Leica) after dissecting the subcutaneous layer, at its point of exit from the stylomastoid foramen, before the facial nerve branches into the main trunk. The main trunk was crushed using a hemostat for 1 min (Fig. 1). This standard crushing procedure ensured that all nerve fibers were injured and the axonal sheath remained intact [18]. The rats were randomly divided into the control (n=7) and study groups (n=7). PBS-soaked (100 μl) gelfoam was topically applied to the crush site in the control group and (100 μl, 2 mg/ml) the study group (Fig. 2).
Evaluation of vibrissa fibrillation
Vibrissa fibrillation in both groups was recorded for 40 s using the iPhone video system 1, 2, 3, and 4 weeks after inducing the crush injury. The frequency of vibrissa fibrillation was analyzed using BORIS (https://www.boris.unito.it), an animal behavior evaluation software. The percentage of vibrissa fibrillation (left side: crush injury/right side: normal) at each week was compared between the control and study groups.
Measurement of the threshold of electrically stimulated muscle action potential
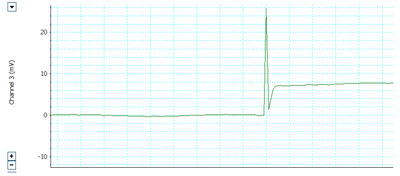
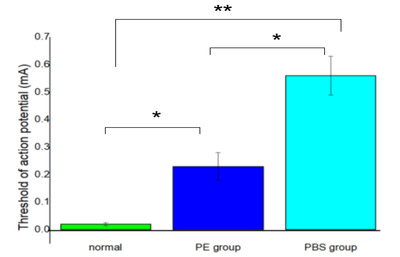
Electrophysiological analysis was conducted before and four weeks after the crush injury. The threshold action potential was measured using the method described in our previous study [29, 30]. The facial nerves on the left side were re-exposed. The right intact site was used as the normal control. The facial nerve monitoring system was equipped with electromyography (AD Instruments, Castle Hill, Australia). The subdermal needle electrodes were positioned percutaneously into the orbicularis oculi, orbicularis oris, and leg (ground needle) to record compound action potential (CMAP) signals, which were evoked by stimulation of the main trunk of the FN directly using a monopolar tungsten probe (Xomed-Treace, Jacksonville, FL, USA). Electrical signals (rectangular current pulse for 0.05 ms) were delivered to the main trunk of the facial nerve using a monopolar stimulating electrode connected to a pulse generator (A-320D; World Precision Instruments Inc., Sarasota, FL, USA). The distance and direction of the monopolar stimulating probe relative to the main trunk of the facial nerve were adjusted using a micromanipulator. All MAPs were measured using maximal nerve stimulation. Data were automatically acquired using the lab chart system (PowerLab; AD Instrument, Castle Hill, Australia), which was displayed on a Samsung computer monitor, and analyzed using the Scope software (AD Instrument). The peak amplitude of the action potential waveform was determined to assess the recovery from facial nerve injury (Fig. 3).
Histological examination
Evaluation using light microscopy
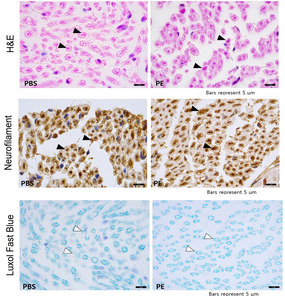
After measurement of the threshold action potential, the distal portion of the main trunk was taken using micro-scissors under a surgical microscope. Segments of nerve tissue sections were carefully dissected and fixed in 4% paraformaldehyde in PBS. The fixed tissues were routinely processed, embedded in paraffin, sectioned into 4-μm thick sections, deparaffinized, and rehydrated using standard protocols. The overall morphology was visualized using routine hematoxylin and eosin (H&E) staining.
Myelin was stained with Luxol Fast Blue (LFB). In brief, the rehydrated tissue sections were incubated in 0.1% LFB solution overnight at 56℃, rinsed with 95% ethyl alcohol and distilled water, differentiated in 0.05% lithium carbonate solution, dehydrated in a series of alcohol solutions, cleared with xylene, and mounted in a resinous medium.
Immunohistochemistry
Axonal microtubules and Schwann cells were visualized via immunohistochemistry using anti-neurofilament and anti-S-100 antibodies, respectively. Briefly, the rehydrated sections were blocked with normal goat serum (Vector ABC Elite Kit; Vector Laboratories) for 1 h, incubated with rabbit anti-neurofilament and anti-S-100 primary antibodies (1:500; Abcam, Cambridge, UK) overnight at 4℃, reacted with biotinylated goat anti-rabbit IgG (Vector ABC Elite Kit) for 2 h at room temperature (RT), reacted with avidin-biotin peroxidase complex (Vector ABC Elite Kit) for 1 h at RT, and developed with diaminobenzidine substrate (DAB kit; Vector Laboratories). The relative staining intensities, average positive cell sizes, and average axonal diameters were analyzed using the ImageJ software (https://imagej.nih.gov/ij/download.html). The parameters are expressed as mean±error (SEs) (n= 5/group).
Evaluation using transmission electron microscope
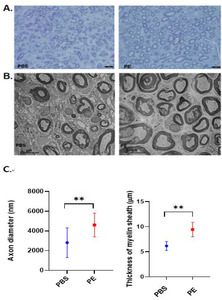
After euthanasia, the distal portion of the facial nerve from two rats in both groups was rapidly excised using micro-scissors and immediately immersed in 2.5% glutaraldehyde fixation for transmission electron microscopy (TEM). Tissue samples were washed with phosphate buffer and post-fixed using 1% osmium tetroxide. After serial dehydration using ethanol, the nerve samples were embedded in a mixture of resins (LR white resin). Transverse semi-thin (5-μm-thick) sections were cut using a microtome, stained with toluidine blue, and observed using light microscopy. Ultrathin sections were cut immediately following a series of semi-thin sections. They were examined using a JEM-2100F field-emission transmission electron microscope (JEM-2100F, JEOL Ltd., Tokyo, Japan). The thickness of the myelin sheath was measured using ImageJ.
Statistical analysis
The in vitro data and threshold of action potential were analyzed using the analysis of variance and Tukey’s multiple comparison test, recovery of vibrissa fibrillation was analyzed by t-test, and thickness of myelin sheath by Mann-Whitney test using GraphPad Prism version 8.01 for Windows (GraphPad Software, CA, USA).
RESULTS
In vitro studies
Proliferation effect of PE in RSC96 Schwann cells
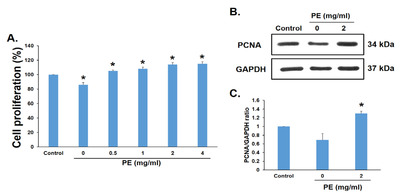
The cells were incubated with PE (0~4 mg/ml) for 24 h to evaluate the proliferative effect of PE, and cell proliferation was measured using the MTT assay; 0 mg/ml represents no addition of PE. The difference between the control and PE groups was that the control consisted of FBS, whereas 0 mg/ml contained neither FBS nor PE. The proliferation of RSC96 cells treated with PE was significantly higher (up to 14%; Fig. 4A). PCNA levels were measured by immunoblot analysis to confirm the effect of PE at the molecular level. PCNA expression increased by 29% in RSC96 cells treated with PE 2 mg/ml (Fig. 4B, C). Thus, the results showed that PE exerted proliferation effects in RSC96 cells.
Migratory effects of PE in RSC96 Schwann cells
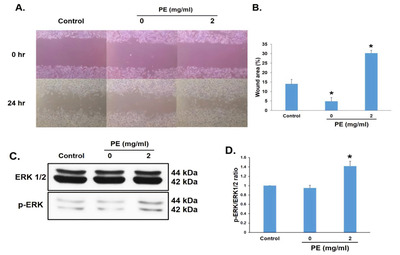
RSC96 cells were incubated with 2 mg/ml PE for 24 h to determine whether PE affected the migratory ability of RSC96 cells. Cell migration was determined using a wound-healing assay. PE treatment significantly increased the relative wound closure rate (Fig. 5A, B) compared with that in the control group. The migration-related protein ERK was measured using immunoblot analysis to confirm its effect at the molecular level. The expression of p-ERK increased in RSC96 cells treated with 2 mg/ml PE (Fig. 5C, D). Thus, these results suggest that PE enhanced the migration of RSC96 cells.
Growth factor secreted by Schwann cells treated with PE
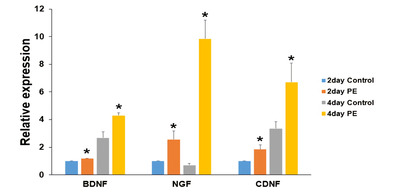
The effects of topical application of 2 mg/ml PE on RSC96 cells were further investigated by detecting the gene expression of important neurotrophic factors such as
In vivo studies
Assessment of vibrissa fibrillation
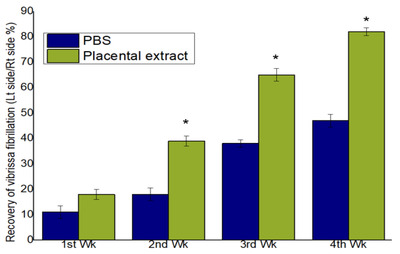
All the rats included in the experiment survived, and no intraoperative complications were observed. Video observations of vibrissa fibrillation and analysis using BORIS showed rapid behavioral improvement in the study group (albeit gradually) compared to that in the control group. No significant difference was observed between the PE and control groups during the 1st week postoperatively. However, a significant improvement was observed at postoperative 2nd, 3rd, and 4th weeks in the study group (p<0.05) (Fig. 7).
Recovery of electrically stimulated muscle action potential threshold
The recovery of the threshold and action potentials differed significantly between the study and control groups at 4 weeks postoperatively. Moreover, there was a significant difference between the PE group and the contralateral and normal sides of the control group (p<0.05) (Fig. 8).
Histological findings
H&E staining and neurofilament immunostaining revealed that the axonal diameter was greater in the PE-treated facial nerves, while LFB staining and S-100 immunostaining revealed more robust and thicker myelin sheaths enveloping the axons in the PE-treated group compared with those in the untreated control group (Fig. 9). These findings suggest that PE can elicit neuronal regeneration after mechanical injury via axonal regeneration and Schwann cell proliferation. The size of the axons was larger in the PE group, and the thickness of the myelin sheath was significantly larger in the PE group (Fig. 10).
DISCUSSION
The regenerative capacity of peripheral nerves is limited and their functional recovery is poor [31]. Permanent complications can occur before reinnervation of the regenerated axons [32]. Interaction between axonal connections of the facial nerve is essential for maintaining facial expression.
The symmetry of the eyes at rest, eye closure ability after the blinking reflex by blowing air onto the cornea, symmetry of the vibrissae (whiskers) at rest relative to the normal central position of the nose tip, and vibrissa fibrillation (motion) compared to the normal or untreated side are used for clinical scoring of mimetic muscle recovery. However, these assessments are subjective. In the present study, we assessed the recovery of vibrissa fibrillation using animal behavior analysis software (BORIS) with video analysis. This method was objective. To the best of our knowledge, this is the first study to analyze the recovery of vibrissa fibrillation in a facial nerve paralysis model.
Gelfoam-impregnated PE was useful for the topical delivery of growth factors contained in PE to the facial nerve following paralysis induced by crush injury. In the present study, PE enhanced vibrissa fibrillation and recovery of the threshold and action potential, which was the result of nerve regeneration, including myelination. PE aided in the regeneration of axonal integrity by remyelination, as identified by the increase in the robustness and thickness of the myelin sheath based on H&E, LFB, neurofilament, S-100 staining, semi-thin section, and TEM findings.
The degenerative process by which injured axons and the myelin sheaths surrounding them are eliminated is called Wallerian degeneration. The removal of myelin debris is an important stage in the regeneration process. Within two days of damage, the distal stump fragments and Schwann cells start to clean the axonal and myelin debris, and the Schwann cells also proliferate and differentiate simultaneously [33]. Within two weeks of entering the distal stump, macrophages and Schwann cells clear the area of debris. Then, led by Schwann cell tubes, the axons regenerate from the proximal stump and make contact with the Schwann cells once more [34].
Schwann cells are the main source of myelin sheath, and myelination is an important property of axons that plays a significant role in neuronal regeneration following nerve injury. Therefore, Schwann cells are important for the development and maintenance of the peripheral nerves. They significantly affect how peripheral nerves develop, work, and are maintained, which helps foster a supportive environment for regeneration. The ability of Schwann cells to proliferate and migrate to the site of injury plays a major role in the repair of peripheral nerve damage. Our in vitro study focused on the effect and mechanism of PE in RSC96 Schwann cells. In this study, a significant increase in the proliferation and migration of Schwann cells in the presence of 2 mg/ml PE was observed. Moreover, the expression of
CONCLUSION
Although further evaluations are needed, our results demonstrated the enhancing effects of PE in the crush injured facial nerve paralysis model based on behavioral, functional, and morphological analyses. PE may be considered as a topical therapeutic agent for treating traumatic facial nerve paralysis.
Supplemental Materials
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C1014176).
This paper was presented as oral presentation in TERMIS-AP 2022 (Tissue Engineering and Regenerative Medicine International Society Asia-Pacific Chapter Conference) October 5-8,2022/ICC Jeju, South Korea.
Figures
Tables
Primers used in the real-time polymerase chain reaction
| Gene | Forward primer (5’→3’) | Reverse primer (5’→3’) | Acc. No. |
|---|---|---|---|
| NGF | CCAAGGACGCAGCTTTCTATC | CTGTGTCAAGGGAATGCTGAAG | NM_001277055.1 |
| CDNF | CGAGGGCTGACTGTGAAGTA | GGTGGCCGAGTCTTTGGTAG | NM_001037543 |
| BDNF | GTGGTTACCTGACTGGGCTC | ACAGGGGATTCAGTGGGACT | NM_001270630.1 |
| GAPDH | CTGCCACTCAGAAGACTGTGG | TTCAGCTCTGGGATGACCTTG | NM_017008.4 |
References
- Gordin E, Lee TS, Ducic Y, Arnaoutakis D (2015) Facial nerve trauma: evaluation and considerations in management. Craniomaxillofac Trauma Reconstr 8:1-13
- Patel A, Lofgren DH, Varacallo M (2021) Temporal fracture. In: StatPearls (In: Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Sedeh PA, eds). StatPearls Publishing, Treasure Island, FL.
- Matsumine H, Sasaki R, Tabata Y, Matsui M, Yamato M, Okano T, Sakurai H (2016) Facial nerve regeneration using basic fibroblast growth factor-impregnated gelatin microspheres in a rat model. J Tissue Eng Regen Med 10:E559-E567
- Wang P, Zhao H, Yao Y, Lu C, Ma J, Chen R, Pan J (2020) Repair of facial nerve crush injury in rabbits using collagen plus basic fibroblast growth factor. J Biomed Mater Res A 108:1329-1337
- Sugiyama M, Ito T, Furukawa T, Hirayama A, Kakehata S (2020) The effect of insulin-like growth factor 1 on the recovery of facial nerve function in a guinea pig model of facial palsy. J Physiol Sci 70:28
- Wang Y, Zhao X, Huojia M, Xu H, Zhuang Y (2016) Transforming growth factor-β3 promotes facial nerve injury repair in rabbits. Exp Ther Med 11:703-708
- Liu H, Wen W, Hu M, Bi W, Chen L, Liu S, Chen P, Tan X (2013) Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen Res 8:3139-3147
- Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle MJ, Weich H, Ahmed A (1996) Localisation of placenta growth factor (PIGF) in human term placenta. Growth Factors 13:243-250. color plates I-II, pre.bk cov
- Hofmann GE, Scott RT Jr, Bergh PA, Deligdisch L (1991) Immunohistochemical localization of epidermal growth factor in human endometrium, decidua, and placenta. J Clin Endocrinol Metab 73:882-887
- Lysiak JJ, Hunt J, Pringle GA, Lala PK (1995) Localization of transforming growth factor beta and its natural inhibitor decorin in the human placenta and decidua throughout gestation. Placenta 16:221-231
- Uehara Y, Kitamura N (1996) Hepatocyte growth factor/scatter factor and the placenta. Placenta 17:97-101
- Zhang D, Lijuan G, Jingjie L, Zheng L, Wang C, Wang Z, Liu L, Mira L, Sung C (2011) Cow placenta extract promotes murine hair growth through enhancing the insulin - like growth factor-1. Indian J Dermatol 56:14-18
- Devi HL, Kumar S, Konyak YY, Bharati J, Bhimte A, Pandey Y, Kumar K, Paul A, Kala A, Samad HA, Verma MR, Singh G, Bag S, Sarkar M, Chouhan VS (2020) Expression and functional role of fibroblast growth factors (FGF) in placenta during different stages of pregnancy in water buffalo (Bubalus bubalis). Theriogenology 143:98-112
- O'Keefe EJ, Payne RE, Russell N (1985) Keratinocyte growth-promoting activity from human placenta. J Cell Physiol 124:439-445
- Ehrig K, Leivo I, Argraves WS, Ruoslahti E, Engvall E (1990) Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci U S A 87:3264-3268
- Watanabe S, Togashi S, Takahashi N, Fukui T (2002) L-tryptophan as an antioxidant in human placenta extract. J Nutr Sci Vitaminol (Tokyo) 48:36-39
- Scatena CD, Adler S (1998) Characterization of a human-specific regulator of placental corticotropin-releasing hormone. Mol Endocrinol 12:1228-1240
- Benham FJ, Povey MS, Harris H (1978) Placental-like alkaline phosphatase in malignant and benign ovarian tumors. Clin Chim Acta 86:201-215
- Schmidt CL, Sarosi P, Steinetz BG, O'Byrne EM, Tyson JE, Horvath K, Sas M, Weiss G (1984) Relaxin in human decidua and term placenta. Eur J Obstet Gynecol Reprod Biol 17:171-182
- Bersinger NA, Groome N, Muttukrishna S (2002) Pregnancy-associated and placental proteins in the placental tissue of normal pregnant women and patients with pre-eclampsia at term. Eur J Endocrinol 147:785-793
- Seo TB, Han IS, Yoon JH, Seol IC, Kim YS, Jo HK, An JJ, Hong KE, Seo YB, Kim DH, Park SK, Yang DC, Namgung U (2006) Growth-promoting activity of Hominis Placenta extract on regenerating sciatic nerve. Acta Pharmacol Sin 27:50-58
- Komobuchi H, Hato N, Teraoka M, Wakisaka H, Takahashi H, Gyo K, Tabata Y, Yamamoto M (2010) Basic fibroblast growth factor combined with biodegradable hydrogel promotes healing of facial nerve after compression injury: an experimental study. Acta Otolaryngol 130:173-178
- Kohmura E, Yuguchi T, Yoshimine T, Fujinaka T, Koseki N, Sano A, Kishino A, Nakayama C, Sakaki T, Nonaka M, Takemoto O, Hayakawa T (1999) BDNF atelocollagen mini-pellet accelerates facial nerve regeneration. Brain Res 849:235-238
- Yasui G, Yamamoto Y, Shichinohe R, Funayama E, Oyama A, Hayashi T, Furukawa H (2016) Neuregulin-1 released by biodegradable gelatin hydrogels can accelerate facial nerve regeneration and functional recovery of traumatic facial nerve palsy. J Plast Reconstr Aesthet Surg 69:328-334
- Yildiz M, Karlidag T, Yalcin S, Ozogul C, Keles E, Alpay HC, Yanilmaz M (2011) Efficacy of glial growth factor and nerve growth factor on the recovery of traumatic facial paralysis. Eur Arch Otorhinolaryngol 268:1127-1133
- Ladage D, Turnbull IC, Ishikawa K, Takewa Y, Rapti K, Morel C, Karakikes I, Hadri L, Müller-Ehmsen J, Costa KD, Hajjar RJ, Kawase Y (2011) Delivery of gelfoam-enabled cells and vectors into the pericardial space using a percutaneous approach in a porcine model. Gene Ther 18:979-985
- Hom DB, Medhi K, Assefa G, Juhn SK, Johnston TP (1996) Vascular effects of sustained-release fibroblast growth factors. Ann Otol Rhinol Laryngol 105:109-116
- Heo JH, Heo Y, Lee HJ, Kim M, Shin HY (2018) Topical anti-inflammatory and anti-oxidative effects of porcine placenta extracts on 2,4-dinitrochlorobenzene-induced contact dermatitis. BMC Complement Altern Med 18:331
- Jang CH, Lee S, Park IY, Song A, Moon C, Cho GW (2019) Memantine attenuates salicylate-induced tinnitus possibly by reducing NR2B expression in auditory cortex of rat. Exp Neurobiol 28:495-503
- Cho G, Moon C, Maharajan N, Ang MJ, Kim M, Jang CH (2022) Effect of pre-induced mesenchymal stem cell-coated cellulose/collagen nanofibrous nerve conduit on regeneration of transected facial nerve. Int J Mol Sci 23:7638
- Silver J, Schwab ME, Popovich PG (2014) Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol 7:a020602
- Cattin AL, Lloyd AC (2016) The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol 39:38-46
- Klein D, Martini R (2016) Myelin and macrophages in the PNS: an intimate relationship in trauma and disease. Brain Res 1641:130-138
- Jessen KR, Mirsky R (2016) The repair Schwann cell and its function in regenerating nerves. J Physiol 594:3521-3531
- Wang H, Zhu H, Guo Q, Qian T, Zhang P, Li S, Xue C, Gu X (2017) Overlapping mechanisms of peripheral nerve regeneration and angiogenesis following sciatic nerve transection. Front Cell Neurosci 11:323





