Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2023; 32(1): 31-41
Published online February 28, 2023
https://doi.org/10.5607/en22036
© The Korean Society for Brain and Neural Sciences
Negative Influence of the Hunger State on Rule-observance Behavior in Mice
Abdelrahman M. Alkahwaji1,2, Hee-Sup Shin1,2* and C. Justin Lee1,2*
1Center for Cognition and Sociality, Institute for Basic Science (IBS), Daejeon 34141, 2IBS School, University of Science and Technology, Daejeon 34141, Korea
Correspondence to: *To whom correspondence should be addressed.
Hee-Sup Shin, TEL: 82-42-878-9101, FAX: 82-42-878-9151
e-mail: shin@ibs.re.kr
C. Justin Lee, TEL: 82-42-878-9150, FAX: 82-42-878-9151
e-mail: cjl@ibs.re.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Developing social strategies to share limited resources equally and maximize the long-term benefits of conflict resolution is critical for appropriate social interactions. During social interactions, social decision-making depends not only on the external environment, but also on internal factors, such as hunger, thirst, or fatigue. In particular, hunger, which is related to food as a physical need, plays a dominant role in social decision-making. However, the consequences of food deprivation on social decision-making are not well understood. We have previously shown that mice with rule-observance behavior are capable of resolving conflict during social decision-making by observing a well-established social strategy based on reward zone allocation. Here, we developed a rule-observance behavior paradigm wherein the hunger state is achieved by applying food restrictions on mice prior to social behavior experiments. We found that the hunger state in mice deteriorated the established social strategy by decreasing reaction time, implying an increase in impulsivity. In contrast, the hunger state did not affect reward zone allocation, indicating no effect on spatial memory. This decrease in reaction time led to a significant increase in the percentage of violations during rule observance and a significant decrease in the amount of reward (payoff equity). Our study proposes that the hunger state exerts a detrimental effect on appropriate social decision-making by decreasing reaction time, increasing violation, and decreasing payoff equity in rule-observance behavior.
Graphical Abstract
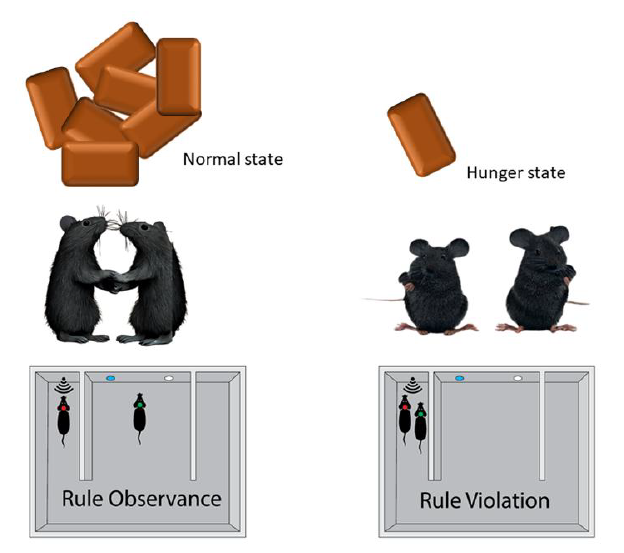
Keywords: Animal behavior, Hunger, Decision-making, Social behavior, Classical conditioning
INTRODUCTION
Food, territory, and well-matched mates are pivotal resources for both humans and animals. Securing stable resources is crucial for survival and social stability. However, this is not easy to achieve. In contrast, aggression and social conflict are risky and costly behaviors [1, 2]. Physical conflict may cause injuries and disability at the bodily level. Additionally, it causes stress and depression at the psychological level. Moreover, mutual violation is time- and resource-intensive. Therefore, developing social strategies to resolve conflicts and share resources equally and wisely is critical for animal survival. Human and non-human animals possess the ability to establish a social strategy for resource sharing and conflict resolution. These strategies depend not only on external factors, such as the environment or availability of resources, but also on internal states, such as hunger, fatigue, and thirst. Previous studies have examined the role of internal states in modifying the behavior of mice when performing several cognitive tasks. For instance, one study found that food availability affects the representation and valuation of choices during decision-making [3]. The hunger state can affect prosocial behavior, such as helping behavior in mice [4]. In addition, fatigue can affects the performance of mice during learning tasks [5]. Moreover, mounting evidence indicates that physiological needs, such as water and food, shape valuation and motivation, and guide decision-making to restore homeostasis [6, 7]. Food, as a physiological need, plays an influential role in decision-making [6, 8]. Despite numerous studies, however, the influence of hunger on social decision-making and social interactions is not well-understood.
The hunger state is known to exert a negative influence on decision-making and motivational states [9]. In addition, the hunger state affects the social state and social decision-making during social games [10]. Delay discounting is an agent’s preference for an immediate reward, even a smaller, short-term reward compared to a larger, long-term one [11]. In humans, hunger has been shown to increase the impact of delay discounting on food and non-food rewards, such as money or music [12]. The hunger state has a detrimental effect on the valuation of economic decision-making [6]. A previous study found a correlation between food deprivation and impulsive responses during decision-making in rodents [13]. An increase in impulsivity negatively affects social cooperation [14]. Hungry impulsive mice cannot make the correct decision [15]. However, the effect of negative impulsivity on the rewards gained and social strategies during social decision-making remains unclear.
To address these issues, we developed a modified form of the previously established rule-observance behavioral paradigm [8]. Using wireless-brain stimulation (WBS) as a rewarding system, we expanded the social conflict resolution test to test the effect of the hunger state on social conflict during rule-observance behavior. We tested the effect of the hunger state on mutual observance and mutual violation pairs. By calculating the observance percentage and violation percentage for each pair in normal and hunger states, we could monitor the influence of the hunger state on the behavior of mice during rule-observance behavior. Because social rule in rule-observance behavior is achieved by reward zone allocation, we tested the reward zone allocation in both normal and hunger states. Additionally, we examined the effect of the hunger state on the reaction time response compared with that in the normal state. These spatial and temporal investigations were performed to understand how the internal state (hunger) affects the behavior of mice during rule-observance behavior.
MATERIALS AND METHODS
Animals and surgery
All mice used in this study were male B57BL/6J mice. Before stereotaxic surgery, the mice were kept in cages containing five mice in a 12:12 light/dark cycle with food and water accessible ad libitum. All animal studies and experimental procedures were approved by the Animal Care and Use Committee of the Institute for Basic Science (Daejeon, Korea).
At eight weeks of age, stereotaxic bipolar electrode (MS303T/2-B/SPC, Plastics One, Roanoke, Virginia) implantation surgery was performed in the right medial forebrain bundle. (-1.2; +1.2; -5.0, AP; ML; DV, in millimeters from the bregma). The mice were anesthetized with intraperitoneally injected ketamine (120 mg/kg). The mice were then fixed in a Kopf stereotaxic set. The skull was exposed and adjusted so that the bregma and lambda were on the same horizontal axis. A drill was used to create holes in the mouse skull for the fixation of screws and bipolar electrodes, which were inserted vertically at the aforementioned coordinates. Finally, the bipolar electrode was fixed using an acrylic resin dental cement. After electrode implantation, each pair of mice was housed together in one cage during the conditioning and social conflict tests, separated by a transparent partition. The location of the electrode was confirmed after the experiment by sacrificing and collecting the brains. After bipolar implantation, the mice were allowed to recover for one week before the conditioning and behavioral experiments.
Rewarding system
During rule-observance behavior testing, we used wireless brain stimulation as a reward system, as previously described [7]. The WBS headset was small (1.5×1.5 cm) and lightweight (1.2 g), and generated an electrical current when it sensed an infrared signal from the external controller. The WBS headset was connected to a bipolar electrode implanted into a part of the reward circuitry in the brain, the medial forebrain bundle (reward circuit). When the mouse enters the reward (payoff) zone, an automatic IR signal is emitted from the transmitter above the reward zone to stimulate the headset receiver to produce an electrical current to stimulate the reward circuit.
Rule-observance behavior
Conditioning
The conditioning and social conflict resolution chamber was a two-armed maze. It consisted of a start zone, two reward (payoff) zones where the mice received WBS when entering these zones, two blue LED lights near the reward zones, speakers, and a camera for mouse detection. This hardware was connected to custom-made software to automatically detect the mice based on the color of the bulb in the headset (red or green). The two arms of the reward zones were separated from the other parts of the chamber by transparent partitions.
In the conditioning phase, only one mouse was trained at a time, and the trial commenced by releasing the mouse into the start zone. One of two blue lights (right or left blue light - cue) turned on indicating the right choice of reward zones (left or right reward zone). If the mouse entered the correct reward zone, the positive reinforcement reward was delivered to the mouse as 5 s of the WBS IR reward signal, and this was regarded as a successful trial. Otherwise, the punishment for negative reinforcement was delivered as a loud noise through the speaker and this was regarded as a failed trial. After the mouse received the reward or punishment, the trial was terminated automatically, and the system returned to its normal state.
The conditioning phase lasted for 20 d. Each day consisted of 20 trials or 40 min each (whichever finished first). The mice were considered conditioned if they performed 100% of the trials in the last three days (60 trials) and the number of successful trials during that period exceeded 45 trials (binomial test: 20 trials, probability of trial=0.5 – p<0.001).
Social conflict resolution test
In the social conflict resolution state, the pair test was performed by combining two well-conditioned mice from the conditioning phase. The social conflict resolution test consisted of 40 trials or 20 min (whichever finished first). The paired test lasted 20 d. The trials were initiated when both mice entered the start zone and one of the two blue LED lights was turned on. The type of trial (observance or violation) was determined based on two scenarios. First, if only one mouse received the reward in the correct arm without any disturbance from the other mouse, we regarded this trial as an observance trial. In contrast, if the other mouse disturbed the 5 s reward period, the reward signal was terminated automatically and this was regarded as a rule violation trial. No negative reinforcement, that is, loud noise, was used in the pair test. This procedure was performed automatically depending on the different colors of the headsets (red and green) using an automatic object detection system and camera. Based on the behavior of the mice, we calculated the degree of rule observance as follows:
Food restriction protocol
After social conflict resolution tests and before food restriction, we aimed to maintain the body weight of the mice at 80% of the initial body weight. To achieve this goal, food provided to the mice was restricted to create a 30% deficit compared to their daily needs while performing food restriction social behavior tests. This allowed the mice to be hungry during social tests. Body weight was monitored daily after the behavioral test. Mice had free access to water. Moreover, in this study, we defined the reaction time as the time from the initiation of the trial (two mice enter the start zone and the blue light turns on) to the time the mice entered the reward zone (IR signal turns on).
Statistical analysis
For all analyses, the D’Agostino-Pearson test was used to check data normality. Differences between the normal and hunger state groups were assessed using paired two-tailed Student’s t-test to analyze the significance of the observance percentage and amount of rewards acquired (following normal distribution). To analyze reward zone allocation and reaction time (not following normal distribution), the Mann-Whitney test was used to analyze the difference between the normal and hunger state groups.
The results are evaluated for significance using asterisks (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n.s., not significant). Outliers were excluded by using the Grubb test. GraphPad Prism 9.3.1 for Windows (GraphPad Software, USA) was used to create the plots.
RESULTS
Classical operant conditioning using Wireless Brain Stimulation (WBS)
Classical operant associative conditioning in rodent behavior is based on the association between animal behavior and reward (positive reinforcement) or punishment (negative reinforcement) with specific cues based on the animal behavioral response [16, 17]. To test the effect of the hunger state on rule-observance behavior in mice, we first trained a mouse to perform the correct tasks by associating the cue (Blue LED light) with the correct reward zone. The experimental timeline is shown in Fig. 1A for surgery, classical conditioning, social conflict resolution, and food restriction. We performed surgery on 50 mice to implant a bipolar electrode. We found that 22 of 50 mice (44%) passed the learning threshold (Table 1) (see Materials and Methods). We found that the conditioning performance of mice commenced at 0.5 successful trials, and steadily increased throughout the conditioning days to reach the threshold value of 0.75 (Fig. 1C). We then examined the time required to complete 20 trials on the conditioning days. During the initial days, an entire 40 min period was required to complete the conditioning paradigm (Fig. 1D). In the progress of conditioning, the time required decreased until it reached 9.7±2.98 min. The time decay was calculated using a nonlinear regression curve (Fig. 1D). These results implied that well-conditioned mice required a short amount of time to complete the conditioning sessions (Fig. 1C, 1D). Of the 8732 conditioning trials, the mice were successful for 63% of the trials and failed 37% of the trials (Fig. 1E). After the conditioning, the mice were sacrificed in order to confirm the position of the silicon probe in the medial forebrain bundle (Fig. 1F). Overall, mice showed efficient learning during operant conditioning in rule-observance behavior.
The effect of hunger on the behavior of mice during rule-observance behavior
Next, we performed a social conflict-resolution test. After 20 days of conditioning, we grouped the 22 well-trained conditioned mice into 11 pairs (pairs distinguished by red and green light bulbs attached to the headset). As an example, we provided the results from one day (day 20) of Pair 4 in the normal state to show the types of trials (observance and violation) (Fig. 2B). In this example, 25 out of 40 trials were observance trials and the remaining 15 were violation trials (Fig. 2B). Based on these data, we calculated the percentage of observance of each mouse with an observance percentage of 65% for left mouse and 60% for right mouse, and with a violation percentage of 35% for left mouse and 40% for right mouse (Fig. 2B). We plotted the average observance and violation trial percentages of the mouse left on the x-axis and the average observance and violation percentages of the mouse right on the y-axis in normal and food restriction states (Figs. 2 C~2E). There was an increase in the number of violation trials along with a consequent decrease in the observance level (Fig. 2E).
After completing the social conflict resolution test, we had 11 pairs of animals, six pairs for mutual rule-observance (observance vs. observance), and two pairs for mutual rule-violation (violation vs. violation), which are represented as blue dots in the upper-right and lower-left quadrants, respectively. And Three pairs were observed and were violated (Fig. 2F). Consequently, we measured the observance percentage in the same 11 pairs in the hungry state. We found that nine pairs became mutual violations, one pair showed both violation observances, and one pair showed mutual observance (Fig. 2G). These data showed a decrease in the observance percentage (Fig. 2H). In addition, we calculated the violation percentage in the normal and hunger states. We found a low violation percentage in the normal state and an increase in violation in the hungry state (Figs. 2I~2K). To ensure an association between the hunger state and deterioration of rule observance behavior, we allowed the mice to access the food pellet (refeeding) as in a normal state after the food restriction state. We observed an increase in the percentage of observance (Fig. 2L). This implies that the hunger state disturbed the established rule observance behavior.
In summary, we tabulated the change in the observance percentage throughout the social testing period in the normal and hunger states. The observance percentage increased during the normal state, but strikingly dropped during the food restriction state and increased after refeeding (Fig. 2L). There was a significant difference in the percentage of observance between the normal and food restriction states (*p-value 0.0168 paired t-test) (Fig. 2M-left), and there was a significant difference between the normal and food restriction states in the amount of reward and payoff equity (**p=0.0019, paired t-test) (Fig. 2M). The percentage of observation trials dropped from 73% in the normal state to 62% in the food restriction state (Fig. 2N). Finally, the hunger state had a detrimental effect on well-established social rules, acquired rewards, and payoff equity.
Spatial and temporal effect on mice behavior during the rule-observance behavior test in a food restriction state
Choe et al. demonstrated that mice could resolve conflict owing to limited resources by splitting the two reward zones using a reward zone allocation strategy [8]. Here, we investigated whether mice utilized the same strategy (reward zone allocation), and found that the mice developed a reward zone allocation strategy in a normal state in most of the mice pairs in normal and food restriction states (Fig. 3A). At the beginning of the pair test, the occupation rate was low, and over consecutive trials, the behavior developed to almost reach the maximum occupation rate in the specified reward zone for both the left and right zones (Fig. 3B, 3C). No significant difference in reward zone allocation was observed between the normal and food restriction states (paired t-test: p=0.1733) (Fig. 3D). To further investigate the underlying pathway that shifts the rule-observance behavior in mice during food restriction states, we compared the reaction times in both states (normal and food restriction states). Analysis of the reaction time in the paired test trials revealed a significant difference in reaction time between the normal group and the food restriction group (Fig. 3E, 3F). The food restriction group showed a faster reaction time than that of the normal group (Non-parametric: Mann-Whitney test ****, p<0.0001). Overall, these findings indicate temporal variation, not spatial variation, in the behavior of mice between normal and hunger states.
DISCUSSION
This study is the first to demonstrate that resource scarcity can disrupt the social contract between animals. We found that the internal state significantly negatively affected the well-established social agreement between mice. We used a previously established rule-observance behavior test [8] as a social decision-making behavioral paradigm to study the effect of an internal state (hunger state). This behavioral paradigm allows us to study decision-making, multi-agent reinforcement learning, social behavior, game theory, and control theory in mice. The social interactions consist of spatial and temporal components [18, 19]. As previously demonstrated, spatial conflict resolution using the reward zone allocation strategy leads to each mouse gaining most of its reward from one of two reward zones (left or right) without interference from the other mouse [8]. In this study, we found that the internal state affected rule-observance behavior without changing reward zone allocation, indicating no change in spatial memory. In contrast, we found that the hunger state led to a decrease in the temporal parameter (reaction time) of rule observance (Fig. 3F). This could be owing to the fact that mice collected considerably less information through sensory cues in the hunger state, resulting in hasty decision-making [20]. This indicates that the hunger state affects the rule-observance behavior of mice by decreasing the reaction time.
The hunger state induces changes in neurotransmitters and neuromodulators that affect aggression
It is well established that hunger and fasting increase aggression in animal species [21, 22]. Hunger is regarded as a catalyst for aggression [23]. The hunger state provokes a release of the hunger hormone ghrelin from the stomach, which passes through the blood-brain barrier. Therefore, although we did not directly measure this in this study, it is possible that an elevated level of ghrelin could be present in the brain and affect rule-observance behavior. It has also been reported that an increase in ghrelin levels by central infusion is sufficient to increase inter-male aggression in mice [24], suggesting that ghrelin could play an important role in hunger-induced aggression. This suggests that ghrelin could be the key mechanistic link between hunger and the behavioral anomalies observed during the rule-observance behavior test conducted in this study. Further investigations are needed to test this possibility.
In addition to hunger-related hormones such as ghrelin, many neurohormones are affected by the hunger state. For instance, previous studies have demonstrated that food restriction significantly decreases hypothalamic and cortical levels of serotonin [25-27]. In addition, a previous study reported a correlation between a decrease in central serotonin levels and an increase in aggression [28]. Furthermore, two separate studies found that the suppression of serotonergic neuronal firing increases the level of aggression in mice [29], and a decrease in serotonin levels caused by depleting its precursor tryptophan increases aggression owing to the prefrontal cortex’s inability to control the emotional response to anger [30]. It is possible that the level of serotonin decreased during the rule-observance behavior test conducted in this study, which subsequently increased aggression and disrupted proper decision-making by reducing reaction time. Further research is required to determine serotonin levels during rule-observance behavior experiments.
Interestingly, we did not observe any physical aggression between the mice during the rule-observance behavior sessions. This discrepancy could be owing to the use of WBS as a reward system in this study, as opposed to using physical rewards, such as food or sugar pellets. The absence of physical rewards may have prevented the induction of physical aggression, as Choe’s [8] study showed an increase in physical aggression in the case of food rewards. Aggression can manifest in many forms other than physical aggression. In the rule-observance behavior test used in this study, aggression manifested in the form of violating well-established rules or disturbing the other mouse's rewards. Such silent aggressive behavior can be considered “passive aggression,” which is a form of concealed aggression. This study describes, for the first time, a behavioral paradigm that can assess a novel form of behavioral aggression in mice.
The controversy over the role of the hunger state in social decision-making
The effect of the hunger state on decision-making and social interaction has remained a controversial topic. Some studies claim that the hunger state decreases social interactions with a male or female intruder [9, 31]. In contrast, other studies report that hunger does not impede prosociality during social decision-making [32]. In addition, prosociality may be affected by the hunger state [4]. Moreover, the hunger state has been shown to have no effect on economic behavior [33]. Interestingly, a previous study reported that the effect of the hunger state on social behavior depends on the type of hunger: acute or chronic [32]. Our study findings are consistent with those of previous studies reporting that the hunger state affects decision-making and social interaction. Our results clearly demonstrate that the hunger state has a detrimental effect on social decision-making (Fig. 2M), and that both acute and chronic hunger states affect decision-making and social interaction (Fig. 2L). Taken together, our study provides compelling evidence to strengthen the idea that the hunger state affects social interaction and decision-making.
In conclusion, this study is the first to demonstrate that a negative internal state (that is, a hunger state) has a detrimental effect on social decision-making with respect to rule-observance behavior. Most previous studies have focused on the effects of external stimuli and sensory input on social decision-making [34, 35]. Little is known about the effects of internal state on social decision-making and animal behavior. We demonstrated that the hunger state can disturb the social contract between freely behaving animals by compelling them to make fast and hasty social decisions. The novel concepts and tools developed in this study will prove useful in delineating the detailed molecular, cellular, and circuit mechanisms of social decision-making in future studies.
ACKNOWLEDGEMENTS
We thank the mouse supply team in Institute of Basic Science (IBS) for their assistance in conducting this study.
Figures



Tables
An example of conditioning (training) data for the 10 mice during the 20 d of conditioning
| Mouse-1 | Mouse-2 | Mouse-3 | Mouse-4 | Mouse-5 | ||||||||||||||||||||
| S | F | Ratio | D | S | F | Ratio | D | S | F | Ratio | D | S | F | Ratio | D | S | F | Ratio | D | |||||
| 1 | 10 | 9 | 0.526 | 40'01'' | 13 | 7 | 0.65 | 27'00'' | 11 | 9 | 0.55 | 32'03'' | 11 | 9 | 0.55 | 23'33'' | 8 | 10 | 0.444 | 40'19'' | ||||
| 2 | 9 | 5 | 0.643 | 40'01'' | 12 | 8 | 0.6 | 24'04'' | 11 | 9 | 0.55 | 39'50'' | 7 | 13 | 0.35 | 38'17'' | 10 | 10 | 0.5 | 36'28'' | ||||
| 3 | 4 | 8 | 0.333 | 40'01'' | 12 | 8 | 0.6 | 30'56'' | 10 | 9 | 0.526 | 40'01'' | 10 | 10 | 0.5 | 31'41'' | 13 | 7 | 0.65 | 30'44'' | ||||
| 4 | 11 | 6 | 0.647 | 40'01'' | 9 | 11 | 0.45 | 24'09'' | 9 | 11 | 0.45 | 33'36'' | 9 | 11 | 0.45 | 35'14'' | 13 | 7 | 0.65 | 17'10'' | ||||
| 5 | 11 | 9 | 0.55 | 37'01'' | 10 | 10 | 0.5 | 15'48'' | 14 | 6 | 0.7 | 28'52'' | 9 | 11 | 0.45 | 23'06'' | 11 | 9 | 0.55 | 26'22'' | ||||
| 6 | 10 | 9 | 0.526 | 40'1'' | 10 | 10 | 0.5 | 14'37'' | 8 | 12 | 0.4 | 18'7'' | 9 | 11 | 0.45 | 25'47'' | 11 | 9 | 0.55 | 26'18'' | ||||
| 7 | 10 | 10 | 0.5 | 24'33'' | 12 | 8 | 0.6 | 18'08'' | 13 | 7 | 0.65 | 29'10'' | 12 | 8 | 0.6 | 26'47'' | 8 | 12 | 0.4 | 23'22'' | ||||
| 8 | 12 | 8 | 0.6 | 28'36'' | 11 | 9 | 0.55 | 11'19'' | 9 | 11 | 0.45 | 22'19'' | 9 | 11 | 0.45 | 22'28'' | 9 | 11 | 0.45 | 24'25'' | ||||
| 9 | 10 | 10 | 0.5 | 35'38'' | 13 | 7 | 0.65 | 8'36'' | 12 | 8 | 0.6 | 13'48'' | 15 | 5 | 0.75 | 32'1'' | 11 | 9 | 0.55 | 23'44'' | ||||
| 10 | 12 | 8 | 0.6 | 21'57'' | 12 | 8 | 0.6 | 6'30'' | 13 | 7 | 0.65 | 11'59'' | 10 | 10 | 0.5 | 17'8'' | 11 | 9 | 0.55 | 18'34'' | ||||
| 11 | 13 | 7 | 0.65 | 30'41'' | 11 | 9 | 0.55 | 8'17'' | 12 | 8 | 0.6 | 12'20'' | 16 | 4 | 0.8 | 15'46'' | 10 | 10 | 0.5 | 18'07'' | ||||
| 12 | 13 | 7 | 0.65 | 12'07'' | 14 | 6 | 0.7 | 8'43'' | 16 | 4 | 0.8 | 19'38'' | 16 | 4 | 0.8 | 13'35'' | 10 | 10 | 0.5 | 26'09'' | ||||
| 13 | 11 | 9 | 0.55 | 14'06'' | 17 | 3 | 0.85 | 8'46'' | 15 | 5 | 0.75 | 13'31'' | 12 | 8 | 0.6 | 10'40'' | 10 | 10 | 0.5 | 19'46'' | ||||
| 14 | 14 | 6 | 0.7 | 15'13'' | 19 | 1 | 0.95 | 7'16'' | 15 | 5 | 0.75 | 12'03'' | 13 | 7 | 0.65 | 9'18'' | 11 | 9 | 0.55 | 16'18'' | ||||
| 15 | 17 | 3 | 0.85 | 15'19'' | 18 | 2 | 0.9 | 6'20'' | 17 | 3 | 0.85 | 11'33'' | 15 | 5 | 0.75 | 8'44'' | 10 | 10 | 0.5 | 30'04'' | ||||
| 16 | 18 | 2 | 0.9 | 15'26'' | 20 | 0 | 1 | 8'42'' | 12 | 8 | 0.6 | 18'22'' | 13 | 7 | 0.65 | 10'29'' | 10 | 10 | 0.5 | 11'13'' | ||||
| 17 | 11 | 9 | 0.55 | 12'54'' | 15 | 5 | 0.75 | 4'42'' | 13 | 7 | 0.65 | 10'07'' | 12 | 8 | 0.6 | 10'5'' | 13 | 7 | 0.65 | 9'39'' | ||||
| 18 | 17 | 3 | 0.85 | 13'55'' | 16 | 4 | 0.8 | 8'18'' | 17 | 3 | 0.85 | 8'46'' | 16 | 4 | 0.8 | 8'12'' | 16 | 4 | 0.8 | 11'12'' | ||||
| 19 | 15 | 5 | 0.75 | 16'45'' | 17 | 3 | 0.85 | 7'54'' | 17 | 3 | 0.85 | 9'46'' | 15 | 5 | 0.75 | 13'27'' | 18 | 2 | 0.9 | 8'10'' | ||||
| 20 | 16 | 4 | 0.8 | 12'33'' | 16 | 4 | 0.8 | 12'43'' | 15 | 5 | 0.75 | 10'06'' | 16 | 4 | 0.8 | 5'23'' | 20 | 0 | 1 | 4'55'' | ||||
| Mouse-6 | Mouse-7 | Mouse-8 | Mouse-9 | Mouse-10 | ||||||||||||||||||||
| S | F | Ratio | D | S | F | Ratio | D | S | F | Ratio | D | S | F | Ratio | D | S | F | Ratio | D | |||||
| 1 | 10 | 10 | 0.5 | 39'17'' | 10 | 10 | 0.5 | 31'58'' | 12 | 8 | 0.6 | 32'56'' | 13 | 7 | 0.65 | 32'22'' | 11 | 9 | 0.55 | 39'23'' | ||||
| 2 | 10 | 8 | 0.556 | 40'1'' | 8 | 12 | 0.4 | 38'23'' | 14 | 6 | 0.7 | 38'03'' | 12 | 8 | 0.6 | 33'14'' | 12 | 8 | 0.6 | 33'24'' | ||||
| 3 | 6 | 14 | 0.3 | 28'33'' | 12 | 8 | 0.6 | 29'18'' | 15 | 5 | 0.75 | 27'53'' | 12 | 8 | 0.6 | 33'14'' | 12 | 8 | 0.6 | 32'24'' | ||||
| 4 | 14 | 6 | 0.7 | 30'58'' | 14 | 6 | 0.7 | 21'20'' | 7 | 13 | 0.35 | 32'41'' | 10 | 10 | 0.5 | 32'2'' | 9 | 11 | 0.45 | 24'15'' | ||||
| 5 | 12 | 8 | 0.6 | 37'52'' | 8 | 12 | 0.4 | 27'43'' | 13 | 7 | 0.65 | 34'28'' | 15 | 5 | 0.75 | 29'12'' | 10 | 10 | 0.5 | 29'35'' | ||||
| 6 | 8 | 12 | 0.4 | 33'11'' | 9 | 11 | 0.45 | 22'59'' | 13 | 7 | 0.65 | 21'3'' | 12 | 8 | 0.6 | 21'33'' | 11 | 9 | 0.55 | 30’15’’ | ||||
| 7 | 8 | 12 | 0.4 | 37'16'' | 9 | 11 | 0.45 | 19'14'' | 13 | 7 | 0.65 | 18'03'' | 12 | 8 | 0.6 | 19'52'' | 9 | 11 | 0.45 | 25'5'' | ||||
| 8 | 9 | 6 | 0.6 | 40' | 13 | 7 | 0.65 | 24'9'' | 11 | 9 | 0.55 | 17'13'' | 10 | 10 | 0.5 | 18'27'' | 13 | 7 | 0.65 | 26'46'' | ||||
| 9 | 8 | 12 | 0.4 | 34'16'' | 9 | 11 | 0.45 | 19'31'' | 11 | 9 | 0.55 | 15'55'' | 13 | 7 | 0.65 | 13'11'' | 12 | 8 | 0.6 | 17'3'' | ||||
| 10 | 11 | 9 | 0.55 | 24'06'' | 11 | 9 | 0.55 | 13'23'' | 13 | 7 | 0.65 | 15'39'' | 12 | 8 | 0.6 | 9'9'' | 12 | 8 | 0.6 | 8'47'' | ||||
| 11 | 9 | 11 | 0.45 | 19'25'' | 11 | 9 | 0.55 | 14'49'' | 9 | 11 | 0.45 | 12'35'' | 13 | 7 | 0.65 | 11'8'' | 11 | 9 | 0.55 | 9'21'' | ||||
| 12 | 11 | 9 | 0.55 | 24'57'' | 9 | 11 | 0.45 | 11'44'' | 10 | 10 | 0.5 | 12'59'' | 17 | 3 | 0.85 | 7'43'' | 11 | 9 | 0.55 | 8'13'' | ||||
| 13 | 10 | 10 | 0.5 | 21'8'' | 10 | 10 | 0.5 | 13'6'' | 13 | 7 | 0.65 | 12'34'' | 15 | 5 | 0.75 | 8'53'' | 13 | 7 | 0.65 | 11'3'' | ||||
| 14 | 12 | 8 | 0.6 | 10'21'' | 10 | 10 | 0.5 | 13'48'' | 12 | 8 | 0.6 | 10'26'' | 17 | 3 | 0.85 | 8'31'' | 10 | 10 | 0.5 | 8'20'' | ||||
| 15 | 13 | 7 | 0.65 | 10'38'' | 13 | 7 | 0.65 | 11'53'' | 15 | 5 | 0.75 | 10'19'' | 16 | 4 | 0.8 | 7'59'' | 13 | 7 | 0.65 | 6'41'' | ||||
| 16 | 12 | 8 | 0.6 | 11'02'' | 19 | 1 | 0.95 | 12'50'' | 15 | 5 | 0.75 | 11'26'' | 19 | 1 | 0.95 | 7'57'' | 15 | 5 | 0.75 | 6'39'' | ||||
| 17 | 15 | 5 | 0.75 | 11'25'' | 15 | 5 | 0.75 | 14'29'' | 13 | 7 | 0.65 | 9'01'' | 18 | 2 | 0.9 | 8'55'' | 13 | 7 | 0.65 | 6'09'' | ||||
| 18 | 18 | 2 | 0.9 | 8'58'' | 15 | 5 | 0.75 | 15'25'' | 16 | 4 | 0.8 | 10'39'' | 15 | 5 | 0.75 | 11'20'' | 15 | 5 | 0.75 | 7'21'' | ||||
| 19 | 19 | 1 | 0.95 | 6'44'' | 16 | 4 | 0.8 | 10'09'' | 16 | 4 | 0.8 | 11'34'' | 15 | 5 | 0.75 | 6'45'' | 15 | 5 | 0.75 | 5'16'' | ||||
| 20 | 17 | 3 | 0.85 | 8'04'' | 20 | 0 | 1 | 10'16'' | 16 | 4 | 0.8 | 12'5'' | 20 | 0 | 1 | 8'12'' | 18 | 2 | 0.9 | 5'48'' | ||||
S, successful trial; F, failed trail; Ratio, ratio of successful to failed trials; D, duration of the conditioning session.
References
- Huntingford FA, Turner AK (1987) Animal conflict. Chapman and Hall, London
- Guo X, Dukas R (2020) The cost of aggression in an animal without weapons. Ethology 126:24-31
- Rangel A, Camerer C, Montague PR (2008) A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci 9:545-556
- Ueno H, Suemitsu S, Murakami S, Kitamura N, Wani K, Matsumoto Y, Okamoto M, Ishihara T (2019) Helping-like behaviour in mice towards conspecifics constrained inside tubes. Sci Rep 9:5817
- Mizunoya W, Oyaizu S, Hirayama A, Fushiki T (2004) Effects of physical fatigue in mice on learning performance in a water maze. Biosci Biotechnol Biochem 68:827-834
- Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM (2015) Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521:180-185
- Eiselt AK, Chen S, Chen J, Arnold J, Kim T, Pachitariu M, Sternson SM (2021) Hunger or thirst state uncertainty is resolved by outcome evaluation in medial prefrontal cortex to guide decision-making. Nat Neurosci 24:907-912
- Choe IH, Byun J, Kim KK, Park S, Kim I, Jeong J, Shin HS (2017) Mice in social conflict show rule-observance behavior enhancing long-term benefit. Nat Commun 8:1176
- Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Brüning JC, Krashes MJ (2016) Hunger-driven motivational state competition. Neuron 92:187-201
- Aarøe L, Petersen MB (2013) Hunger games: fluctuations in blood glucose levels influence support for social welfare. Psychol Sci 24:2550-2556
- Steward T, Mestre-Bach G, Fernández-Aranda F, Granero R, Perales JC, Navas JF, Soriano-Mas C, Baño M, Fernández-Formoso JA, Martín-Romera V, Menchón JM, Jiménez-Murcia S (2017) Delay discounting and impulsivity traits in young and older gambling disorder patients. Addict Behav 71:96-103
- Skrynka J, Vincent BT (2019) Hunger increases delay discounting of food and non-food rewards. Psychon Bull Rev 26:1729-1737
- Viana DS, Gordo I, Sucena E, Moita MA (2010) Cognitive and motivational requirements for the emergence of cooperation in a rat social game. PLoS One 5:e8483
- Stephens DW, McLinn CM, Stevens JR (2006) Effects of temporal clumping and payoff accumulation on impulsiveness and cooperation. Behav Processes 71:29-40
- Edman G, Schalling D, Levander SE (1983) Impulsivity and speed and errors in a reaction time task: a contribution to the construct validity of the concept of impulsivity. Acta Psychol (Amst) 53:1-8
- Xiao X, Deng H, Furlan A, Yang T, Zhang X, Hwang GR, Tucciarone J, Wu P, He M, Palaniswamy R, Ramakrishnan C, Ritola K, Hantman A, Deisseroth K, Osten P, Huang ZJ, Li B (2020) A genetically defined compartmentalized striatal direct pathway for negative reinforcement. Cell 183:211-227.e20
- Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, Shenasa MA, Johnson AB, Fife KH, Faget L, Hnasko TS (2016) Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun 7:13697
- Carstensen LL, Isaacowitz DM, Charles ST (1999) Taking time seriously. A theory of socioemotional selectivity. Am Psychol 54:165-181
- Hoppler SS, Segerer R, Nikitin J (2022) The six components of social interactions: actor, partner, relation, activities, context, and evaluation. Front Psychol 12:743074
- Heitz RP, Schall JD (2012) Neural mechanisms of speed-accuracy tradeoff. Neuron 76:616-628
- Fokidis HB, Prior NH, Soma KK (2013) Fasting increases aggression and differentially modulates local and systemic steroid levels in male zebra finches. Endocrinology 154:4328-4339
- Janson C, Vogel E (2006) Hunger and aggression in capuchin monkeys. In: Feeding ecology in apes and other primates (Hohmann G, Robbins MM, Boesch C, eds), pp 283-310. Cambridge University Press, New York, NY
- Rohles FH Jr, Wilson LM (1974) Hunger as a catalyst in aggression. Behaviour 48:123-130
- Vestlund J, Winsa-Jörnulf J, Hovey D, Lundström S, Lichtenstein P, Anckarsäter H, Studer E, Suchankova P, Westberg L, Jerlhag E (2019) Ghrelin and aggressive behaviours-evidence from preclinical and human genetic studies. Psychoneuroendocrinology 104:80-88
- Haider S, Haleem DJ (2000) Decreases of brain serotonin following a food restriction schedule of 4 weeks in male and female rats. Med Sci Monit 6:1061-1067
- Haleem DJ, Haider S (1996) Food restriction decreases serotonin and its synthesis rate in the hypothalamus. Neuroreport 7:1153-1156
- van Erp AM, Miczek KA (2000) Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci 20:9320-9325
- Coccaro EF (1989) Central serotonin and impulsive aggression. Br J Psychiatry Suppl 8:52-62
- Audero E, Mlinar B, Baccini G, Skachokova ZK, Corradetti R, Gross C (2013) Suppression of serotonin neuron firing increases aggression in mice. J Neurosci 33:8678-8688
- Passamonti L, Crockett MJ, Apergis-Schoute AM, Clark L, Rowe JB, Calder AJ, Robbins TW (2012) Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol Psychiatry 71:36-43
- Burnett CJ, Funderburk SC, Navarrete J, Sabol A, Liang-Guallpa J, Desrochers TM, Krashes MJ (2019) Need-based prioritization of behavior. Elife 8:e44527
- Häusser JA, Stahlecker C, Mojzisch A, Leder J, Van Lange PAM, Faber NS (2019) Acute hunger does not always undermine prosociality. Nat Commun 10:4733
- Elbæk CT, Mitkidis P, Aarøe L, Otterbring T (2022) Honestly hungry: acute hunger does not increase unethical economic behavior. J Exp Soc Psychol 101:104312
- Waskom ML, Kiani R (2018) Decision making through integration of sensory evidence at prolonged timescales. Curr Biol 28:3850-3856.e9
- Luczak A, Bartho P, Harris KD (2013) Gating of sensory input by spontaneous cortical activity. J Neurosci 33:1684-1695





