Articles
Article Tools
Supplementary
Stats or Metrics
Article
Original Article
Exp Neurobiol 2023; 32(2): 68-82
Published online April 30, 2023
https://doi.org/10.5607/en23002
© The Korean Society for Brain and Neural Sciences
A Critical Involvement of Glutamatergic Neurons in the Anterior Insular Cortex for Subdiaphragmatic Vagotomy-induced Analgesia
Yea Jin Kim1, Grace J Lee1, Sang Wook Shim1, Doyun Kim2 and Seog Bae Oh1,2,3*
1Department of Brain and Cognitive Sciences, College of Natural Sciences, Seoul National University, Seoul 03080,
2Tooth-Periodontium Complex Medical Research Center, School of Dentistry, Seoul National University, Seoul 03080,
3Department of Neurobiology & Physiology, School of Dentistry and Dental Research Institute, Seoul National University, Seoul 03080, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-740-8656, FAX: 82-2-762-5107
e-mail: odolbae@snu.ac.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
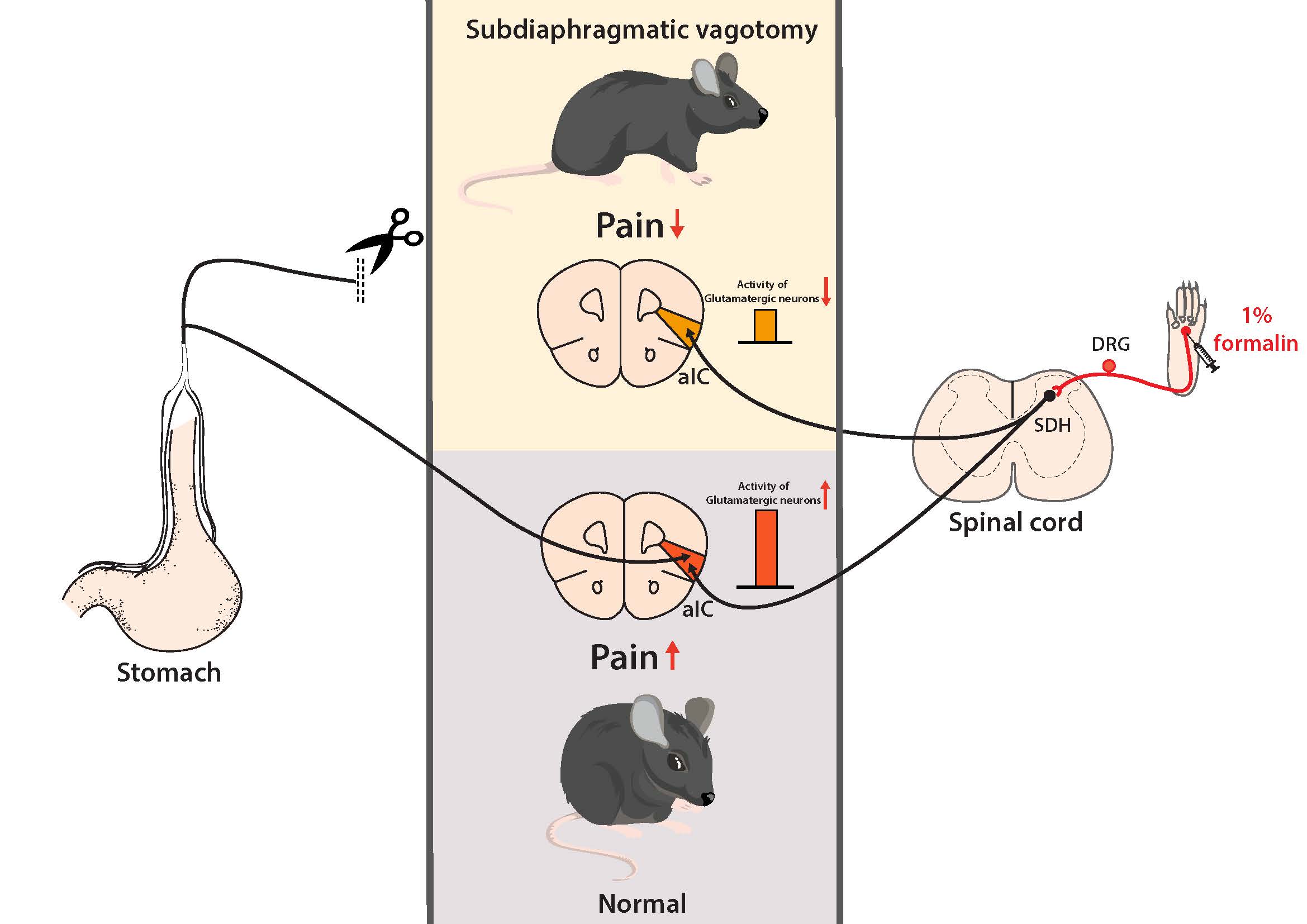
Subdiaphragmatic vagotomy (SDV) is known to produce analgesic effect in various pain conditions including not only visceral pain but also somatic pain. We aimed to determine brain mechanisms by which SDV induces analgesic effect in somatic pain condition by using formalin-induced acute inflammatory pain model. We identified brain regions that mediate SDV-induced analgesic effect on acute inflammatory pain by analyzing c-Fos expression in the whole brain. We found that c-Fos expression was specifically increased in the anterior insular cortex (aIC) among subregions of the insular cortex in acute inflammatory pain, which was reversed by SDV. These results were not mimicked in female mice, indicating sexual-dimorphism in SDV-induced analgesia. SDV decreased c-Fos expressions more preferentially in glutamatergic neurons rather than GABAergic neurons in the aIC, and pharmacological activation of glutamatergic neurons with NMDA in the aIC inhibited SDV-induced analgesic effect. Furthermore, chemogenetic activation of glutamatergic neurons in the aIC reversed SDV-induced analgesia. Taken together, our results suggest that the decrease in the neuronal activity of glutamatergic neurons in the aIC mediates SDV-induced analgesic effect, potentially serving as an important therapeutic target to treat inflammatory pain.
Graphical Abstract
Keywords: Subdiaphragmatic vagotomy (SDV), Acute inflammatory pain, Anterior insular cortex, Glutamatergic neuron
INTRODUCTION
Pain is one of the most prevalent symptoms in various diseases and a common health problem worldwide [1, 2]. Pain is mainly treated with medications, which are sometimes combined with therapies to change lifestyles especially for the management of chronic pain patients [3, 4]. When medications are proven to be ineffective for pain patients, several surgical interventions may help to control pain in certain pain conditions [5]. Interestingly, it has been reported that vagotomy relieves pain in some patients and subdiaphragmatic vagotomy (SDV) attenuates pain under pathological conditions in adult rodents [6-10].
SDV is a surgery to dissect the bilateral vagus nerve that is responsible for the bidirectional communication nerve relaying information between the gut and the brain [11]. Clinical studies have demonstrated that SDV reduces gastric acid in the stomach and attenuates pain in patients with a stomach ulcer or upper gastrointestinal neoplasms [7, 8]. Also, a preclinical study showed that SDV partially suppresses visceral pain induced by a functional dyspepsia model [9]. While the effect of SDV has been well studied with focus on visceral disease and is critically associated with visceral pain [6, 9, 12], recent studies have demonstrated that SDV can relieve somatic pain as well [8, 13]. We also confirmed SDV-induced analgesia in formalin-induced acute inflammatory pain condition [14]. Given SDV attenuates pain originated from extraterritorial area which is not innervated by vagus nerve, supraspinal mechanisms are highly likely to contribute to SDV-induced analgesia in somatic pain conditions.
SDV commonly accompanies the changes in the brain because vagus nerve mainly relays visceral information from the gastrointestinal tract to the brain [11]. Indeed, SDV produces changes of brain-derived neurotrophic factor and corticotrophin-releasing hormone in the brain region associated with pain under visceral pain condition [9]. Moreover, SDV reduces pro-inflammatory gene expression in brain regions such as the basolateral amygdala (BLA) and central amygdala (CeA) in LPS-induced inflammatory pain condition [15]. We also observed that SDV produces pain reduction only in the second phase, not the first phase, of formalin test under acute inflammatory pain condition [14], which is consistent with a previous report [13]. As the second phase of formalin test is known to be mediated by central mechanisms [16, 17], brain mechanisms might be also involved in SDV-induced analgesia. However, it is not fully understood how the brain mechanisms underlie SDV-induced analgesia in somatic pain conditions.
Insular cortex (IC) is a crucial region mainly recognizing the information arising from the stomach [18]. Therefore, SDV blocks the activation of the IC induced by lithium chloride injection which causes visceral stimuli [12]. In addition, the IC is closely involved in pain perception [19], and especially the anterior insular cortex (aIC), a subarea of IC, is highly implicated in inflammatory pain [20]. We also showed that c-Fos expression is significantly enhanced in the aIC by formalin-induced somatic acute inflammatory pain [21]. Furthermore, the glutamatergic signaling in the IC plays a crucial role in modulating pain perception [22]. Given these observations, we hypothesized that the glutamatergic neurons in the aIC may contribute to SDV-induced analgesic effect in the acute inflammatory pain condition.
In the current study, we thus focused on the involvement of the aIC in SDV-induced analgesic effect, by employing the behaviors test, immunohistochemical analysis, pharmacological and chemogenetic manipulations. Here, we elucidate the involvement of glutamatergic neurons in the aIC for SDV-induced analgesia in acute inflammatory pain, which may provide a potential brain therapeutic target for the treatment of inflammatory pain.
MATERIALS AND METHODS
Animals
Both Male and female C57BL/6 mice weighing 20~25 g (5 to 8-week-old) were used for the experiment and purchased from DooYeol Biotech (South Korea). All animals were maintained (5 per cage) in a temperature-controlled room (23±1℃), and a 12-12 h light/dark cycle with standard lab chow (pellet diet) and water
Bilateral subdiaphragmatic vagotomy (SDV)
The procedure of bilateral subdiaphragmatic vagotomy was performed as follows. The mice were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg,
Formalin test
Before the behaviors test, mice were placed in a white acrylic chamber before experiment for two hours for three consecutive days to allow them to adapt to their surroundings. To observe the hind paw, a mirror was placed at a 45° angle below the chamber.
We used the formalin-induced acute inflammatory pain model as previously described [26]. 20 μl of 1% formalin (formaldehyde solution, 36~38%, Junsei, Japan) was injected subcutaneously into the plantar surface of the left hind paw with a 0.3 ml insulin syringe. After formalin injection, the mice were immediately placed in the test chamber and recorded using a video camera for 40 minutes. The time of licking and flinching was measured during each 5 minutes.
Administration of drugs
N-methyl-D-aspartate receptor (NMDA receptor agonist; M2004, MO, USA) was purchased from Sigma-Aldrich. For activation of the aIC, NMDA (0.2 or 1 μg/μl) was diluted in 0.9% normal saline. The concentration (0.1, 0.5 and 1.0 μg/side) of NMDA for activation of the aIC was determined according to previous studies [27-29]. However, we used lower than 1.0 μg of NMDA since our pilot study confirmed that mice were died within 10 minutes after systemic administration of 1.0 μg.
Clozapine-N-oxide (CNO) was purchased from Tocris. To activate DREADD (Designer Receptors Exclusively Activated by Designer Drugs)-expressing neurons in the anterior insular cortex, CNO 10 mg/kg was dissolved in 0.9% normal saline and intraperitoneally injected 30 minutes before the formalin test for systemic injection.
Stereotaxic surgeries
To activate glutamatergic neurons with NMDA in the aIC, C57BL/6 mice were anesthetized by pentobarbital (50 mg/kg,
After the formalin tests were concluded, mice were perfused with 0.1M phosphate-buffered saline (PBS; pH 7.4) and 4% paraformaldehyde (PFA), and the brains were harvested for histologic identification of specific protein and confirmation of correct injection placement in the aIC.
The following vector was used for single-site designer receptors exclusively activated by designer drugs (DREADD) pAAV-CaMKIIa-hM3D(Gq)-mCherry (50476, Addgene, MA, USA). Viral vector was injected at the rate of 0.1 μl/min, with a 10 min of diffusion time. The injection volume for single-site injections was 0.5 μl. Incorrect cannula or virus injections were excluded from the data analysis. DREADD expression was allowed to accumulate for 2 weeks before CNO injections.
Immunohistochemistry
To identify c-Fos expression using DAB immunohistochemistry after anesthetization with the administration of pentobarbital (50 mg/kg,
To detect NeuN and c-Fos expression, sections were incubated for 1 hour in blocking buffer (0.3% Triton X-100, 5% normal donkey serum in PBS) at room temperature. The primary antibodies are chicken anti-NeuN (1:400 dilution, ABN78, Millipore, MA, USA) and rabbit anti-c-Fos (1:400 dilution, ab190289, Abcam, Cambridge, UK) were diluted in the blocking solution, and the sections were incubated for one day at 4℃. The sections were washed several times for 10 minutes each in 0.1 M PBS. The secondary antibodies A488 donkey anti-chicken (1:400 dilution, 703-545-155, Jackson, PA, USA), Cy3 donkey anti-rabbit (1:400 dilution, 711-165-152, Jackson, PA, USA), and DAPI (1:1,000 dilution, D9545, Sigma Aldrich, MO, USA) were diluted in 0.1 M PBS and incubated 1 hour at RT. The slices were then washed several times for 10 minutes with 0.1 M PBS and mounted using VECTASHIELD mounting media (Vector Laboratories, CA, USA).
To visualize CaMKII expression, brain sections were incubated for 1 hour in blocking solution (0.3% Triton X-100, 2% bovine serum in 0.1 M PBS) at room temperature. The primary antibodies mouse anti-CaMKII (1:200 dilution, MA1-048, Thermofisher, MA, USA) and rabbit anti-c-Fos (1:400 dilution, ab190289, Abcam, Cambridge, UK) were diluted in the blocking solution, and the sections were incubated for 48 hours at 4℃. The sections were washed three times for 10 minutes each in 0.1 M PBS. The sections were incubated for 2 hours in biotinylated goat anti-mouse (BP-9200, Vector laboratories, CA, USA) at RT. After 2 hours, the sections were washed three times for 10 minutes with 0.1 M PBS. Then, the sections were processed with the secondary antibody Streptavidin+Cy3 (1:200 dilution, SA-1300, Vector Laboratories, CA, USA), Alexa 488 goat anti-rabbit (1:300 dilution, 111-545-003, Jackson, PA, USA), and DAPI (1:1,000 dilution, D9542, Sigma Aldrich, MO, USA) in 0.1 M PBS for 2 hours at RT. The sections were then washed three times for 10 minutes with 0.1 M PBS and mounted using VECTASHIELD mounting media (Vector Laboratories, CA, USA).
To identify GAD67 expression, brains were cut in a section of 20 μm. The tissues were incubated for an hour in blocking solution (0.3% Triton X-100, 8% normal donkey serum in PBS) at RT. The primary antibodies mouse anti-GAD67 (1:400 dilution, ab26116, Abcam, Cambridge, UK) and rabbit anti-c-Fos (1:400 dilution, ab190289, Abcam, Cambridge, UK) were diluted in the blocking solution for 24 hours at RT. After treatment of primary antibodies, the tissues were washed three times for 10 minutes with 0.1 M PBS. The secondary antibodies FITC donkey anti-rabbit (1:400 dilution, 711-095-152, Jackson, PA, USA), Cy3 donkey anti-mouse (1:400 dilution, 715-165-150, Jackson, PA, USA), and DAPI (1:1,000 dilution, D9542, Sigma Aldrich, MO, USA) were diluted in 0.1 M PBS and incubated for an hour. The tissues were washed several times for 10 minutes with 0.1 M PBS and mounted using VECTASHIELD mounting media (Vector Laboratories, CA, USA).
To verify SDV using tracer, extracted brains from Fluoro-Gold injected mice were post-fixed by 4% PFA and cryoprotected in 30% sucrose solution at 4℃ each overnight. The brains were embedded in OCT compound (4583, Sakura, CA, USA) and frozen and cut at -22℃ in a section of 30 μm. The sections were dehydrated with 70, 95, 100% ethanol and pure Neoclear (109843, Sigma-Aldrich, MO, USA). All tissues were covered with hardening mounting medium (03989, Sigma-Aldrich, MO, USA).
Image analysis
Mounted slides were examined under the bright-field microscope (DM5000B, Leica, Germany). All images were taken at 10x magnification with identical lighting intensity and color balance conditions. To analyze c-Fos expression within the brain region of interest, we collected 4 to 6 sections per mouse (n=3~6 each group). All visible c-Fos positive neurons were counted, and the mean value was used as representative counts. Using Image J software (National Institutes of Health), the images were converted to greyscale, background subtracted, sharped and enhanced contrast, and intensity threshold was adjusted. Expression of c-Fos protein was analyzed for the following regions: the distance from the bregma in the rostrocaudal plane is from +1.98 to 1.6 mm for the anterior insular cortex (aIC) and medial prefrontal cortex (mPFC), +0.61 mm for medial insular cortex (mIC), from +1.4 to 1.0 mm for the anterior cingulate cortex (ACC), nucleus accumbens core (NAcC) and shell (NAcS), from -0.11 mm to -0.23 mm for the posterior insular cortex (pIC), -1.22 mm for the basolateral amygdala (BLA), central amygdala (CeA), lateral hypothalamus (LH) and ventromedial hypothalamus (VMH), -5.02 mm for the ventrolateral periaqueductal gray (vlPAG), lateral parabrachial nucleus (lPBN) and rostral ventromedial medulla (RVM), and -6.96 mm for the nucleus tractus solitaries (NTS). The locations of the brain regions were previously described [21].
All immunofluorescent-stained sections were imaged on a confocal microscope (LSM 700, Carl Zeiss, Germany). We collected six sections per mouse (n=5 mice each group), and all images were taken at 200× or 400× magnification. Image analysis was performed manually by identifying and counting CaMKII+ and GAD67+ in the same area.
Statistical analysis
The priori power analysis was performed to decide the minimal sample size needed to obtain a statistical power of 0.8 at an alpha level of 0.05. In the formalin test, trial test was conducted to determine the effect of SDV or sham on formalin-induced acute inflammatory pain condition. For the resource equation, E can be decided by following formula: E=Total number of animals-Total number of groups. The numerical value of E should be between 10 and 20. After observing that SDV group produced a significant decrease in nocifensive behaviors of second phase after formalin injection, we measured the minimal meaningful effect size (1.764) using the means (172.86 for SDV and 314.43 for sham group in total nocifensive behavior time of second phase) with standard deviation (93.51 for SDV and 97.55 for sham group). Hence, we conducted a power analysis using G*power 3.1 (Faul, University of Kiel, Kiel, Germany) with effect size revealed a sample size of 5 per experimental group. Because of 10~20% attrition rate, we used 6~9 mice per group on behaviors test. Statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, CA, USA). Comparison between two groups was made using the unpaired Student’s t-test. For multiple comparisons, data were analyzed using the one-way or two-way ANOVA followed by the post hoc Bonferroni test. Detailed statistics for each experiment were shown in the figure legend. Data are presented as mean±SEM. Differences with p<0.05 were considered significant.
RESULTS
SDV suppresses nocifensive behaviors in formalin-induced acute inflammatory pain
We first confirmed whether SDV indeed produces the analgesic effect on spontaneous nocifensive behaviors in the acute inflammatory pain condition. We used formalin model, which is widely used to study acute inflammatory pain in many animal models [14, 26, 30]. A formalin test was performed one week after SDV or sham surgery in the male adult mice (Fig. 1A). Consistent with previous studies including our recent work [13, 14], we observed that SDV significantly suppressed nocifensive behaviors during the second phase, not the first phase, of the formalin test (Figs. 1B, C).
SDV reverses enhancement of c-Fos expression by formalin injection in the aIC
We next examined the brain area involved in SDV-induced analgesia via monitoring immunoreactivity (IR) of c-Fos, a marker for neuronal activation in the whole brain including the aIC (Fig. 2A, Supplementary Fig. 2). We found that formalin injection increased c-Fos expression in the aIC, which was significantly reversed by SDV compared to the sham group (Figs. 2A, C). These results suggest that the aIC might be critically involved in SDV-induced analgesic effect in acute inflammatory pain. As shown in Supplementary Fig. 2, we also observed that formalin injection increased c-Fos expression in other brain regions such as the anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC). However, we have not further examined these regions in this study. Three subregions in the IC along the rostro-caudal axis that is divided into the anterior (aIC), medial (mIC) and posterior insular cortex (pIC) are closely associated with pain perception [31]. We thus also examined patterns of c-Fos expression in other subregions (i.e., mIC and pIC) of the insular cortex between the formalin-treated sham and SDV groups. Unlike in aIC, the number of c-Fos expression in the mIC and pIC showed no significant change between the formalin-treated sham and SDV group (Figs. 2B, C). Taken together, these results demonstrated the potential role of the aIC in SDV-induced analgesic effect.
SDV-induced analgesic effect is specific to male mice
We next asked whether SDV-induced analgesic effect is sex-specific and we repeated behavioral tests in the female mice under formalin-induced acute inflammatory pain condition (Fig. 3A). Unlike male mice, the results showed that the nocifensive behaviors did not change with SDV in female (Figs. 3B, C). We also analyzed c-Fos expression in the female group to determine brain regions which showed neuronal activation following SDV. In contrast to results in male mice, the number of c-Fos expression in the aIC was similar between formalin-treated SDV group and the formalin-treated sham group (Fig. 3D). Interestingly, we observed a dramatic increase of c-Fos expression in the ACC and mPFC of the formalin-treated SDV group compared to the formalin-treated sham group (Figs. 3E, F). Overall, these results indicated that sex difference exists in SDV-induced analgesic effect under acute inflammatory pain condition, and also suggest that differential neuronal activities in the brain regions might be associated with sexual-dimorphism in SDV-induced analgesia.
SDV decreases activation of glutamatergic neurons in the aIC under acute inflammatory pain condition
It has been demonstrated that the insular cortex contains 73% of glutamatergic neurons and 27% of GABAergic neurons [32]. We thus explored whether the effect of SDV induces changes of activity in glutamatergic or GABAergic neuron within the aIC by double immunostaining with their specific markers and c-Fos expression. Glutamatergic and GABAergic neurons were confirmed with fluorescent immunostaining of calcium calmodulin kinase II (CaMKII) and glutamic acid decarboxylase 67 (GAD67), respectively (Fig. 4A) [33, 34]. As indicated by colocalization of c-Fos and CaMKII (Figs. 4B, C), the number of co-localized glutamatergic neurons was decreased in the aIC of formalin-treated SDV group. In contrast, SDV did not affect the number of c-Fos colocalized with GAD67 in the aIC (Figs. 4D, E). These results imply that reduced activity of glutamatergic neurons in the aIC contributes to SDV-induced analgesic effect.
NMDA microinjection into the aIC reverses SDV-induced analgesic effect in acute inflammatory pain
To verify involvement of glutamatergic neurons in the aIC in SDV-induced analgesic effect, we employed a pharmacological experiment with NMDA to activate glutamatergic neurons in the aIC. Mice were implanted by cannula in the aIC to administrate NMDA (0.1 or 0.5 μg) or vehicle as shown in Fig. 5A. We examined the injection site by Evans blue into the cannula at the end of experiments and excluded incorrect microinjector placement from data (Fig. 5B). We observed that microinjection of NMDA increased the number of c-Fos expression in the aIC (Fig. 5C). The dose was determined based on previous studies and our pilot result [27-29]. Although the effect of NMDA was minimal with 0.1 μg concentration, 0.5 μg NMDA significantly increased nocifensive behaviors in the second phase of formalin test (Figs. 5D, E). These results indicated that activation of glutamatergic neurons in the aIC reverses SDV-induced analgesic effect.
Chemogenetic activation of glutamatergic neurons in the aIC reverses SDV-induced analgesia in the acute inflammatory pain model
Lastly, we further investigated whether glutamatergic neurons in the aIC mediate SDV-induced analgesia via chemogenetic tool. In this time, we chemogenetically activated glutamatergic neurons in the aIC of male SDV group. Mice were transduced with the hM3Dq receptor via injection of AVV expressing CaMKIIa-mCherry into the aIC (Figs. 6A, B). Virus expression was confirmed via fluorescence imaging (Fig. 6C), and chemogenetic activation of glutamatergic neurons by CNO was confirmed by the level of c-Fos expression after CNO injection (Fig. 6D). We found that CNO infusion reversed SDV-induced analgesia in formalin test (Figs. 6E, F). These results imply that activation of glutamatergic neurons in the aIC reverses SDV-induced analgesia.
DISCUSSION
In this study, we demonstrated critical involvement of the aIC in SDV-induced analgesia in acute inflammatory pain. We further confirmed that decrease in neuronal activities of glutamatergic neurons in the aIC, which are enhanced under acute inflammatory pain condition, contributes to SDV-induced analgesia. Thus, our work delineates a brain mechanism responsible for SDV-induced analgesic effect.
The subregions of the IC play differential roles in pain perception [35] because the pIC preferably participates in the sensory-discriminative pain, and the aIC is mainly involved in the affective-motivational pain [36, 37]. Especially, many studies have documented relationship between affective pain component and increased activities of the aIC [20, 38, 39]. Also, a previous report strongly supports the notion that analgesic effect is closely linked to the reduction in neural activation of the aIC [40]. We observed that SDV reversed prominent increase of c-Fos expression in acute inflammatory pain condition only in the aIC among the subregions of the IC (Fig. 2). Our results clearly indicate that decrease in neuronal activities of aIC which are enhanced under acute inflammatory pain condition contributes to SDV-induced analgesia. It seems that SDV might ameliorate affective pain components rather than discriminative pain by reducing neuronal activities in the aIC. In line with this, it has been reported that SDV also induces decreased inner anxiety behaviors [41] and depression-like behaviors [9, 41, 42]. Thus, SDV might improve negative emotion including affective pain via its effect on the aIC [43].
The aIC is a critical brain region which modulates the sense of the internal state in the body (i.e., interoception) and receives the signal from the stomach [18, 44]. A clinical research showed that the brain regions which receive nutrient signals from the gastrointestinal tract also modulate pain and vagotomy decreases the neuronal activities of these brain regions [45]. Indeed, this report suggests that the analgesic effect can be induced by vagotomy which reduces the brain region's activity from the gastrointestinal tract. Our results indicate that the aIC is one of the brain regions affected by vagotomy. However, when we analyzed c-Fos expressions of the whole brain as an unbiased approach to determine brain areas involved in SDV-induced analgesia, we observed a profound change of neural activity in the ACC and mPFC in addition to aIC (Supplementary Fig. 2) - the decreased c-Fos expression in the ACC and increased c-Fos expression in the mPFC in the formalin-treated SDV group compared to the formalin-treated sham group. The pattern of c-Fos expression in the ACC was comparable to that of the aIC (Fig. 2A, Supplementary Fig. 2). Reduced neural activity in the ACC is associated with analgesic effect [46], and the connectivity between the ACC and aIC is well known to modulate pain, especially in affective-dimension pain [19, 47]. The ACC might be another target which mediates SDV-induced analgesia. Additionally, SDV has been reported to increase limbic neurotransmitters, such as dopamine, in the mPFC, which is also related to pain modulation [48, 49]. The neuronal activity of the mPFC increases by synaptic input of dopaminergic neurons from the ventral tegmental area (VTA) [50]. Increased c-Fos expression in the mPFC in the formalin-treated SDV group (Supplementary Fig. 2) might be due to the enhanced dopaminergic inputs from VTA following SDV. However, further studies are still required to elucidate cellular and molecular mechanisms by which loss of neural projections from the stomach by SDV produces analgesic effects in extraterritorial somatic areas by modulating various brain areas.
The IC is activated during acute and chronic pain in human [51] and rodent [52-54]. Since glutamatergic neuron accounts for a major subtype of neurons in the IC [32], elevated glutamate within the IC has been used for the marker for the presence of central sensitization or pain centralization [55-58]. Additionally, the pain produces increase of the excitatory synaptic transmission in the aIC [20]. Therefore, it can be suggested that glutamatergic neuron in the aIC is activated by noxious stimuli, and modulation of glutamatergic neurons in the aIC is a favored strategy for regulating pain [59]. As examples related to this suggestion, the reduced glutamatergic signaling within the IC decreases nocifensive pain behaviors in chronic pain [22], and specifically chemogenetic inhibition of glutamatergic neurons in the aIC produces analgesic effect in pain condition [20]. In consistent with these studies, we also observed that SDV reduced activity of glutamatergic neurons in the aIC (Fig. 4C), and activation of glutamatergic neurons in the aIC elicited pain behaviors via pharmacological manipulation with NMDA and chemognetics tool in SDV-operated mice (Figs. 5, 6). Therefore, glutamatergic neurons in the aIC may be critically involved in the SDV-induced analgesia.
It was interesting to note that SDV-induced analgesia was not observed in female mice (Fig. 3). In consistent with our results, SDV and gonadectomy suppressed the nocifensive behaviors in the second phase of the formalin test in the female mice, while the administration of estrogen increased nocifensive behaviors [13]. This report suggests that estrogen affects analgesic effect of SDV in the female mice. It has been shown that intravenous (i.v.) injection of estrogen induces significant activation of the insular cortex [60]. This might explain why we did not observe decreased c-Fos expression in the aIC following SDV in the female mice (Fig. 3D). We also found different patterns of c-Fos expression in the ACC between male and female mice (Fig. 3E, Supplementary Fig. 2), which might also contribute to sexual-dimorphism in SDV-induced analgesia.
Vagus nerve stimulation (VNS) has been also shown to have short-term analgesic effect [61, 62]. Studies have indicated that VNS activates various pain-modulating brain regions, including the locus coeruleus, raphe nuclei and periaqueductal gray (PAG) [63-66]. The neurotransmitters norepinephrine, serotonin, GABA, and opioids have been also implicated as possible neurotransmitters of VNS-induced analgesic effect [67]. It seems paradoxical that both stimulation and deprivation of vagal inputs can lead to similar behavioral results. However, our results indicate that brain areas involved in the short-term analgesic effects of SDV (i.e. aIC, ACC and mFPC) differ from those involved in VNS-induced analgesia. Further studies are required to clarify the difference in underlying mechanisms between the VNS- and SDV-induced analgesic effect.
SDV entails structural and functional changes in the brain, including the nucleus of the solitary tract (NTS) [45, 48, 68]. A previous study has demonstrated that total anterograde tracer arising from the left cervical vagus to the NTS was decreased until 10 days of SDV, whereas the number of tracer was similar to that of control group at 30 days following SDV [68]. Therefore, we designed all experiments to be completed within 10 days following SDV when the effect of SDV could be most clearly observed and also verified the success of SDV surgery by comparing stomach expansion and retrograde tracer labeling of DMN of the vagus nerve between sham and SDV-operated mice (Fig. 1A, Supplementary Figs. 1F, G) [23].
In conclusion, this study indicates that SDV suppresses nocifensive behaviors via reduction of the neuronal activity of glutamatergic neurons in the aIC under formalin-induced acute inflammatory pain condition (Fig. 7). Therefore, our results provide the aIC as a new alternative for therapeutic target to modulate inflammatory pain.
Supplemental Materials
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation of Korea grant (NRF-2021R1A2C3003334, NRF-2018R1A5A2024418) funded by the Korean government MSIT (Ministry of Science and ICT). We thank Sehee Kang for illustrating figures.
AUTHORS' CONTRIBUTIONS
YJK designed the research, performed all of experiments, data analysis, figure visualization and wrote the manuscript draft. GJL taught subdiaphragmatic vagotomy surgery. SWS and DYK helped immunohistochemical experiments. SBO supervised the research and extensively edited manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Figures







References
- Nahin RL (2015) Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 16:769-780
- Goldberg DS, McGee SJ (2011) Pain as a global public health priority. BMC Public Health 11:770
- De Gregori M, Muscoli C, Schatman ME, Stallone T, Intelligente F, Rondanelli M, Franceschi F, Arranz LI, Lorente-Cebrián S, Salamone M, Ilari S, Belfer I, Allegri M (2016) Combining pain therapy with lifestyle: the role of personalized nutrition and nutritional supplements according to the SIMPAR Feed Your Destiny approach. J Pain Res 9:1179-1189
- Park HJ, Moon DE (2010) Pharmacologic management of chronic pain. Korean J Pain 23:99-108
- Rothemeyer SJ, Enslin JMN (2016) Surgical management of pain. SAMJ 106:858-860
- Zurowski D, Nowak Ł, Wordliczek J, Dobrogowski J, Thor PJ (2012) Effects of vagus nerve stimulation in visceral pain model. Folia Med Cracov 52:57-69
- Ajao OG (1977) Vagotomy for relief of pain in some upper gastrointestinal neoplasms. J Natl Med Assoc 69:655-658
- Oi M, Kobayashi K (1963) Vagotomy as a surgical procedure for relief of pain. Am J Surg 106:49-56
- Cordner ZA, Li Q, Liu L, Tamashiro KL, Bhargava A, Moran TH, Pasricha PJ (2021) Vagal gut-brain signaling mediates amygdaloid plasticity, affect, and pain in a functional dyspepsia model. JCI Insight 6:e144046
- Nogueira PJ, Tomaz C, Williams CL (1994) Contribution of the vagus nerve in mediating the memory-facilitating effects of substance P. Behav Brain Res 62:165-169
- Breit S, Kupferberg A, Rogler G, Hasler G (2018) Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry 9:44
- Qian K, Liu J, Cao Y, Yang J, Qiu S (2021) Intraperitoneal injection of lithium chloride induces lateralized activation of the insular cortex in adult mice. Mol Brain 14:71
- Khasar SG, Isenberg WM, Miao FJ, Gear RW, Green PG, Levine JD (2001) Gender and gonadal hormone effects on vagal modulation of tonic nociception. J Pain 2:91-100
- Lee JY, Lee GJ, Nakamura A, Lee PR, Kim Y, Won CH, Furue H, Oh SB (2020) Involvement of cannabinoid type 1 receptor in fasting-induced analgesia. Mol Pain 16:1744806920969476
- Ondicova K, Tillinger A, Pecenak J, Mravec B (2019) The vagus nerve role in antidepressants action: efferent vagal pathways participate in peripheral anti-inflammatory effect of fluoxetine. Neurochem Int 125:47-56
- Savage S, Ma D (2015) Experimental behaviour testing: pain. Br J Anaesth 114:721-724
- Dubuisson D, Dennis SG (1977) The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4:161-174
- Levinthal DJ, Strick PL (2020) Multiple areas of the cerebral cortex influence the stomach. Proc Natl Acad Sci U S A 117:13078-13083
- Bushnell MC, Ceko M, Low LA (2013) Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14:502-511
- Bai Y, Ma LT, Chen YB, Ren D, Chen YB, Li YQ, Sun HK, Qiu XT, Zhang T, Zhang MM, Yi XN, Chen T, Li H, Fan BY, Li YQ (2019) Anterior insular cortex mediates hyperalgesia induced by chronic pancreatitis in rats. Mol Brain 12:76
- Lee GJ, Kim YJ, Lee K, Oh SB (2021) Patterns of brain c-Fos expression in response to feeding behavior in acute and chronic inflammatory pain condition. Neuroreport 32:1269-1277
- Watson CJ (2016) Insular balance of glutamatergic and GABAergic signaling modulates pain processing. Pain 157:2194-2207
- Mordes JP, el Lozy M, Herrera MG, Silen W (1979) Effects of vagotomy with and without pyloroplasty on weight and food intake in rats. Am J Physiol 236:R61-R66
- De Jonghe BC, Horn CC (2008) Chemotherapy-induced pica and anorexia are reduced by common hepatic branch vagotomy in the rat. Am J Physiol Regul Integr Comp Physiol 294:R756-R765
- Uemura N, Yagi H, Uemura MT, Hatanaka Y, Yamakado H, Takahashi R (2018) Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol Neurodegener 13:21
- Lee JY, Lee GJ, Lee PR, Won CH, Kim D, Kang Y, Oh SB (2019) The analgesic effect of refeeding on acute and chronic inflammatory pain. Sci Rep 9:16873
- Alijanpour S, Zarrindast MR (2020) Potentiation of morphine-induced antinociception by harmaline: involvement of μ-opioid and ventral tegmental area NMDA receptors. Psychopharmacology (Berl) 237:557-570
- Medeiros P, Negrini-Ferrari SE, Palazzo E, Maione S, Ferreira SH, de Freitas RL, Coimbra NC (2019) N-methyl-D-aspartate receptors in the prelimbic cortex are critical for the maintenance of neuropathic pain. Neurochem Res 44:2068-2080
- Spuz CA, Tomaszycki ML, Borszcz GS (2014) N-methyl-D-aspartate receptor agonism and antagonism within the amygdaloid central nucleus suppresses pain affect: differential contribution of the ventrolateral periaqueductal gray. J Pain 15:1305-1318
- Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC, Betley JN (2018) A neural circuit for the suppression of pain by a competing need state. Cell 173:140-152.e15
- Gehrlach DA, Weiand C, Gaitanos TN, Cho E, Klein AS, Hennrich AA, Conzelmann KK, Gogolla N (2020) A whole-brain connectivity map of mouse insular cortex. Elife 9:e55585
- Ju A, Fernandez-Arroyo B, Wu Y, Jacky D, Beyeler A (2020) Expression of serotonin 1A and 2A receptors in molecular- and projection-defined neurons of the mouse insular cortex. Mol Brain 13:99
- Berg L, Eckardt J, Masseck OA (2019) Enhanced activity of pyramidal neurons in the infralimbic cortex drives anxiety behavior. PLoS One 14:e0210949
- Liu XB, Murray KD (2012) Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia 53 Suppl 1:45-52
- Lu C, Yang T, Zhao H, Zhang M, Meng F, Fu H, Xie Y, Xu H (2016) Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci Bull 32:191-201
- Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655-666
- Benarroch EE (2019) Insular cortex: functional complexity and clinical correlations. Neurology 93:932-938
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004) Neural systems supporting interoceptive awareness. Nat Neurosci 7:189-195
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, Fan J (2012) Anterior insular cortex is necessary for empathetic pain perception. Brain 135(Pt 9):2726-2735
- Kim MJ, Tanioka M, Um SW, Hong SK, Lee BH (2018) Analgesic effects of FAAH inhibitor in the insular cortex of nerve-injured rats. Mol Pain 14:1744806918814345
- Klarer M, Arnold M, Günther L, Winter C, Langhans W, Meyer U (2014) Gut vagal afferents differentially modulate innate anxiety and learned fear. J Neurosci 34:7067-7076
- Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y, Wang X, Hashimoto K (2021) A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun 94:318-326
- Jasmin L, Burkey AR, Granato A, Ohara PT (2004) Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol 468:425-440
- Wang X, Wu Q, Egan L, Gu X, Liu P, Gu H, Yang Y, Luo J, Wu Y, Gao Z, Fan J (2019) Anterior insular cortex plays a critical role in interoceptive attention. Elife 8:e42265
- Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Kondoh T, Uneyama H, Torii K (2009) Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterology 137:262-273
- Takeda R, Watanabe Y, Ikeda T, Abe H, Ebihara K, Matsuo H, Nonaka H, Hashiguchi H, Nishimori T, Ishida Y (2009) Analgesic effect of milnacipran is associated with c-Fos expression in the anterior cingulate cortex in the rat neuropathic pain model. Neurosci Res 64:380-384
- Gu X, Hof PR, Friston KJ, Fan J (2013) Anterior insular cortex and emotional awareness. J Comp Neurol 521:3371-3388
- Kobrzycka A, Napora P, Pearson BL, Pierzchała-Koziec K, Szewczyk R, Wieczorek M (2019) Peripheral and central compensatory mechanisms for impaired vagus nerve function during peripheral immune activation. J Neuroinflammation 16:150
- Ong WY, Stohler CS, Herr DR (2019) Role of the prefrontal cortex in pain processing. Mol Neurobiol 56:1137-1166
- Chung AS, Miller SM, Sun Y, Xu X, Zweifel LS (2017) Sexual congruency in the connectome and translatome of VTA dopamine neurons. Sci Rep 7:11120
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463-484
- Becerra L, Chang PC, Bishop J, Borsook D (2011) CNS activation maps in awake rats exposed to thermal stimuli to the dorsum of the hindpaw. Neuroimage 54:1355-1366
- Borsook D, Becerra L (2011) CNS animal fMRI in pain and analgesia. Neurosci Biobehav Rev 35:1125-1143
- Mao J, Mayer DJ, Price DD (1993) Patterns of increased brain activity indicative of pain in a rat model of peripheral mononeuropathy. J Neurosci 13:2689-2702
- Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE (2012) Reduced insular γ-aminobutyric acid in fibromyalgia. Arthritis Rheum 64:579-583
- Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ (2009) Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum 60:3146-3152
- Gutzeit A, Meier D, Froehlich JM, Hergan K, Kos S, V Weymarn C, Lutz K, Ettlin D, Binkert CA, Mutschler J, Sartoretti-Schefer S, Brügger M (2013) Differential NMR spectroscopy reactions of anterior/posterior and right/left insular subdivisions due to acute dental pain. Eur Radiol 23:450-460
- Gussew A, Rzanny R, Erdtel M, Scholle HC, Kaiser WA, Mentzel HJ, Reichenbach JR (2010) Time-resolved functional 1H MR spectroscopic detection of glutamate concentration changes in the brain during acute heat pain stimulation. Neuroimage 49:1895-1902
- Zhuo M (2016) Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience 338:220-229
- Saleh TM, Connell BJ, Legge C, Cribb AE (2004) Estrogen attenuates neuronal excitability in the insular cortex following middle cerebral artery occlusion. Brain Res 1018:119-129
- Ren K, Randich A, Gebhart GF (1988) Vagal afferent modulation of a nociceptive reflex in rats: involvement of spinal opioid and monoamine receptors. Brain Res 446:285-294
- Ren K, Randich A, Gebhart GF (1991) Effects of electrical stimulation of vagal afferents on spinothalamic tract cells in the rat. Pain 44:311-319
- Basbaum AI, Fields HL (1978) Endogenous pain control mechanisms: review and hypothesis. Ann Neurol 4:451-462
- Randich A, Aicher SA (1988) Medullary substrates mediating antinociception produced by electrical stimulation of the vagus. Brain Res 445:68-76
- Ren K, Randich A, Gebhart GF (1990) Modulation of spinal nociceptive transmission from nuclei tractus solitarii: a relay for effects of vagal afferent stimulation. J Neurophysiol 63:971-986
- Nishikawa Y, Koyama N, Yoshida Y, Yokota T (1999) Activation of ascending antinociceptive system by vagal afferent input as revealed in the nucleus ventralis posteromedialis. Brain Res 833:108-111
- Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, Treig T, Stefan H, Ramsay RE, Wernicke JF, Wilder BJ (1995) Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res 20:221-227
- Peters JH, Gallaher ZR, Ryu V, Czaja K (2013) Withdrawal and restoration of central vagal afferents within the dorsal vagal complex following subdiaphragmatic vagotomy. J Comp Neurol 521:3584-3599





