Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2011; 20(3): 144-152
Published online September 30, 2011
https://doi.org/10.5607/en.2011.20.3.144
© The Korean Society for Brain and Neural Sciences
Antiallodynic Effects of Electroacupuncture Combined with MK-801 Treatment through the Regulation of p35/p25 in Experimental Diabetic Neuropathy
Hye Suk Hwang#, Eun Jin Yang#, Sang Min Lee, Soon Cheol Lee and Sun-Mi Choi*
Division of Standard Research, Korea Institute of Oriental Medicine, Daejeon 305-811, Korea
Correspondence to: #These authors equally contributed to this work.
*To whom correspondence should be addressed.
TEL: 82-42-868-9485, FAX: 82-42-863-9464
e-mail: smchoi@kiom.re.kr
The anti-allodynic effect of NMDA receptor antagonist and acupuncture treatments were explored through spinal p35 regulation of diabetic neuropathic rat. We evaluated the change over time of p35/p25 protein levels in the spinal cord compared with behavioral responses to thermal and mechanical stimulation in streptozotocin (STZ)-induced diabetic rats. Additionally, we studied p35 expression when electroacupuncture (EA) and a sub-effective dose of NMDA (N-methyl-D-aspartate) receptor antagonist (MK-801) were used to treat hyperalgesia in the diabetic neuropathic pain (DNP). Thermal paw withdrawal latency (PWL) and mechanical paw withdrawal threshold (PWT) were significantly decreased in the early stage of diabetes in rats. p35 expression after STZ injection gradually decreased from 1 week to 4 weeks compared to normal controls. p25 expression in 4-week diabetic rats was significantly higher than that of 2-week diabetic rats, and thermal PWL in 4-week diabetic rats showed delayed responses to painful thermal stimulation compared with those at 2 weeks. EA applied to the SP-9 point (2 Hz frequency) significantly prevented the thermal and mechanical hyperalgesia in the DNP rat. Additionally, EA combined with MK-801 prolonged anti-hyperalgesia, increased p35 expression, and decreased the cleavage of p35 to p25 during diabetic neuropathic pain. In this study we show EA combined with a sub-effective dose of MK-801 treatment in DNP induced by STZ that is related to p35/p25 expression in spinal cord.
Keywords: diabetic neuropathic pain, electroacupuncture, MK-801, p35/p25
INTRODUCTION
Diabetic neuropathic pain remains an unmet clinical problem and is poorly relieved by conventional analgesics. Although antidepressant and antiepileptic agents have been shown to be partially effective, clinical studies have reported the difficulty of managing pain caused by these neuropathies (Sindrup and Jensen, 1999). Although N-methyl-d-aspartate (NMDA) receptors antagonists are highly effective in reducing neuropathic pain, these agents cause severe side effects at therapeutic doses, which limit their clinical uses (Chen et al., 2009). The activation of NMDA receptors and the influx of Ca2+ into the postsynaptic cells through the receptor are important for the induction of long-term synaptic plasticity, including long-term potentiation (Li et al., 2001). The impairment of synaptic plasticity in Streptozotocin (STZ)-induced diabetic rats can be linked to an inappropriate level of NMDA receptor stimulation required for the induction phase of long-term potentiation (Grzeda and Wisniewska, 2008).
It is well known that the p35 plays a pivotal role in the nervous system. However, the underlying mechanism of neuropathic hypoalgesia remains poorly understood. The peripheral inflammation has been resulted in the cleavage of p35 to p25, which forms a more stable complex with cyclin-dependent kinase 5 (Cdk5) (Pareek et al., 2006). p35/p25 are prominently expressed in primary sensory and dorsal horn neurons of adult rats, and p35/p25-associated Cdk5 kinase activity in primary sensory and dorsal horn neurons increased following complete Freund's adjuvant (CFA) treatment (Yang et al., 2007). Activation of mitogen-activated protein kinase (MAPK) in nociceptive neurons leads to pain hypersensitivity (Daulhac et al., 2006; Tsuda et al., 2008), and Cdk5/p35 is involved in altering the MAPK pathway (Sharma et al., 2002). Moreover the NMDA receptor and P/Q type voltage-dependent calcium channel (Li et al., 2001) are also phosphorylated by CDK5 and control calcium influx during sensation of pain (Petrenko et al., 2003).
In the present study, we identified the expression of p35 in the spinal dorsal horn according to allodynia development and characterized whether p35 could be a target molecule for synergistic treatment of an NMDA antagonist with electroacupuncture (EA). Moreover, we tested the hypothesis that synergic treatment with an NMDA antagonist along with electroacupuncture produces analgesic effects greater than either agent alone in a maintained diabetic neuropathic pain phase, and we identified alterations in p35 level in response to analgesic effects.
MATERIALS AND METHODS
Experiments were performed on young adult male Sprague-Dawley rats (300~320 g, Koatech, Gyeonggi-do, South Korea). Animals were housed in groups of two in plastic cages with soft bedding and were provided free access to food and water under a 12/12 hour (h) reversed light-dark cycle. All animals were acclimated for 7 days before the experiment began. Animal experiments were carried out in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals, and experimental procedures were approved by the Institutional Animal Care and Use Committee at the Korea Institute of Oriental Medicine.
Experimental diabetes was induced in rats by a single injection of STZ solution (50 mg/kg, i.v.) into the tail vein. STZ was prepared in saline (0.9% NaCl adjusted to pH 4.0 with hydrochloric acid) on ice; the solution was discarded if bubbling was noted. Age-matched control rats for behavioral experiments received an equal volume of saline. We began checking blood sugar levels 48 hours after injections using a blood glucometer (Accu-check Active; Roche Diagnostics, Indianapolis, IN). Rats with values of > 400 mg/dl were considered hyperglycemic and were included in the experimental group (Chen et al., 2009).
Thermal Allodynia: The Hargreaves test (Hargreaves et al., 1988) was used to determine the presence of thermal hyperalgesia by measuring foot withdrawal latency to heat stimulation. Each rat was placed in a Plexiglas® cubicle box (17'22'14 cm) on the glass platform and acclimated for 10 minutes before heat stimulation. The temperature of the glass was measured and maintained at 25±1℃. A heat source (IITC Life Science, CA) was focused to the plantar side of the hind-paw, which was flush against the glass, and a radiant thermal stimulus was delivered to that site. The latency in seconds from the start of heat application until the animal's escape (or after 20 sec. to prevent tissue damage) from the heat stimulus was recorded as paw withdrawal latency (PWL). Paw withdrawal latency was measured to the nearest 0.01 second (sec).
The intensity of the heat stimulus was maintained at a constant level throughout all experiments. The elicited paw movement occurred at a latency of approximately 9~12 sec in control rats. In the preliminary experiments, the intensity of radiant heat was set at 20%, and the latency readings were very variable. The intensity was later adjusted to 40% to obtain more consistent latency readings. The testing was repeated 3 times in 1 animal with an interval of 5 minutes between each application. After the test, animals were returned to their respective cages. Before STZ injection, the animals were acclimated to the apparatus for 1 week and then tested to establish a baseline reference for post-STZ injection readings. Measurements of threshold to heat stimulus were conducted at 3, 5, and 7 days and 2, 3, and 4 weeks after STZ or saline injection in all groups. For the rats receiving EA treatments after pretreatment with NMDA receptor antagonists, postoperative tests were conducted 2, 4, and 6 hours after EA treatment.
Mechanical allodynia: The sensitivity to touch stimuli was assessed by using the dynamic plantar aesthesiometer (Ugo Basile, Varese, Italy), an automated apparatus based on the von Frey filament principle. Rats were placed in Plexiglas cubicles on a wire mesh platform (17'22'14 cm) for 10 minutes to acclimatize, a stimulating probe was positioned under their hind paw, and an increasing vertical force (continuous increase from 0 to 50 g in 10 sec) was applied to the paw. The instrument registered the force intensity that triggered limb withdrawal, and the mean of three readings was used as the tolerance threshold. Measurements of threshold to mechanical stimulus and postoperative tests were conducted in the same manner as those of heat stimulus. We used 2 weeks-rats after STZ-treatment for EA or/and MK-801 treated experimental animal.
EA was applied to the acupuncture points SP9 or ST36 with a pair of bipolar stimulation electrodes after placing the rats under isoflurane anesthesia (in a flow of a mixture of oxygen and nitrous oxide; 3% for induction and 1.5% for maintenance). Two stainless steel acupuncture needles (0.15 mm diameter; DB needle CO, LTD. Korea) were mounted in a holder with 1-mm separation between the tips. The needle set was vertically inserted to a depth of 4 mm (cutaneous and muscle) at the SP9 or ST36 point. These two sites are equivalent to known human acupuncture points: the SP9 ('Yinlingquan') and ST36 ('Zusanli') which are terms designated by the World Health Organization (1993). The SP9 point is located in the inferior border of the medial condyle of the tibia, in the depression between the posterior border of the tibia and gastrocnemius muscle on the medial leg. The ST36 point is located one fingerbreadth lateral to the tibia's anterior crest, 9.0909 cm (3 cun : 1 cun = 3.0303 cm) inferior to ST 35 in the depression to the lateral side of the patella on the leg. Electrical stimulation was performed using a Pulse Generator PG-306 (Suzuki Iryoki Co. Japan) connected to the pair of needle electrodes. Biphasic pulse electrical stimuli 0.25 ms width with 2- or 120-Hz frequencies were applied at an intensity of the muscle twitch threshold (the muscle twitch threshold was about 0.01 mA) to the acupuncture point. The current delivered was monitored at all times, and the total duration of EA stimulation was 30 min. After the termination of EA, anesthesia was immediately discontinued, and the rats usually resumed full activity within 5 min.
Rats were deeply anesthetized by pentobarbital (100 mg/kg, i.p.) and perfused transcardially with 150 ml of phosphate-buffered saline (PBS; composition in mM: NaCl 137, KCl 2.7, KH2PO4 1.5, NaH2PO4 8.1; pH 7.4). We removed L4, L5, and L6 segments of the lumbar spinal cord and prepared specimens of dorsal horn area separated from ventral area of lumbar spinal cord. As spinal dorsal horn, but not ventral area, is mainly responsible for pain transmission. The spinal dorsal horn was mechanically homogenized in 400 ml of ice-cold lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 0.1% SDS, pH 7.5) containing protease inhibitors (10 mg/ml of aprotinin, 1 mmol/l PMSF and 10 mg/ml of leupeptin), agitated at 4℃ for 30 min, and then centrifuged at 13,000 rpm at 4℃ for 30 min. The protein concentration of tissue lysates was determined with a BCA protein assay kit (Interchim, Paris, France). Proteins were size fractionated on a 15% SDS-PAGE gel at 120 V for 2 h. After electrophoresis, proteins were transferred onto a nitrocellulose transfer membrane (Whatman GmbH) at 200 mA for 120 min using the Bio-Rad transfer system. Membranes were blocked with Tris-buffered saline buffer (TBS), pH 7.4, and 5% skimmed milk, then incubated overnight at 4℃ with rat monoclonal anti-p35 (1 : 1,000 dilution, Santa Cruz, USA), or mouse anti-alpha-tubulin (1 : 5,000, AbCAM, Cambridge, UK) in TBS. Alpha-tubulin was used as an internal control. The membrane was then washed for 30 min in a shaking bath, with the wash buffer (TBS buffer containing 0.1% Tween-20) changed every 10 min. The membranes were subsequently incubated with a horseradish peroxidase-conjugated anti-mouse (1 : 1,000, BD Transduction Laboratories) or rabbit (1 : 1,000 dilution, Santa Cruz, USA) IgG antibody for 1 h at room temperature. The washes were repeated before the membrane was developed with a light-emitting, nonradioactive method using ECL reagent (Amersham Inc., Buckinghamshire, UK). The membrane was then subjected to autoluminography for 1~5 min using the LAS-3000 imaging system (Fuji-film, Tokyo, Japan). The relative optical densities of the respective bands were quantified by densitometric scanning of the blots using the LAS-3000 imaging system.
Intrathecal injections of MK-801 (Sigma-Aldrich, USA) or vehicle (saline) were performed under isoflurane anesthesia (4% induction, 2% maintenance), as described previously (Mestre et al., 1994). In brief, the anesthetized rat was held in one hand by the pelvic girdle, and a 30-gauge, 1.25-inch needle connected to a 10 µl Hamilton syringe was inserted into the subarachnoid space between lumbar vertebrae L5 and L6 until a tail-flick was elicited. The syringe was held in position for few seconds after the injection of a volume of 10 µl/rat. Intrathecal injection of vehicle had no effect on nociceptive thresholds/behavior, protein expression and protein phosphorylation. We tested the effective dose (0.001, 0.005, 0.01 mg/rat, n=3) of MK-801 to hyperalgesia, and 0.005 mg/rat was determined to be a sub-effective dose.
All data are expressed as mean±standard error of the mean (SEM). Statistical significance was assessed using two-way repeated-measures analysis of variance (ANOVA) followed by the Student-Newman-Keul's test as a post-hoc test. Statistical significance was taken at p<0.05.
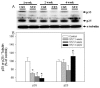
In rats that had been injected intravenously with STZ (50 mg/kg) to remove pancreatic β cells and induce insulin deficiency, the concentration of blood glucose was markedly increased on day 3 (405.5±10.48 mg/dl, p<0.001) and remained stable through the fourth week recorded. In contrast, saline-injected animals maintained normal blood glucose levels (115.1±3.4 mg/dl) that did not differ from baseline levels (Fig. 1A). Thermal paw withdrawal latency (PWL) in diabetic rats significantly decreased at 3 (6.7±0.46 sec) to 21 (6.72±0.65 sec) days after the STZ treatment, but PWL was prolonged on 4 weeks compared with those on 2 week diabetic mice. Mechanical paw withdrawal threshold decreased gradually from day 3 (31.3±2.4 g, p<0.05), with the maximum decrease reached around day 5 (25.5±3.1 g, p<0.001). The diabetes-induced mechanical hyperalgesia persisted until at least the fourth week that we recorded.
Immunoblot (Fig. 2A) and quantification (Fig. 2B) analysis demonstrated the expression of p35 and its cleavage form p25 at 2, 3, and 4 weeks after STZ injection. p35 expression after STZ injection gradually decreased from 1 week (70.05±2.05%) to 4 weeks (58.6±7.07%) compared to normal controls. p25 expression was significantly higher in 4-week diabetic rats than in 2-week diabetic rats. Controls were age-matched normal rats compared with STZ-injected rats, and the quantification of the controls was indicated by the sum of the control blot from 1 week to 4 weeks.
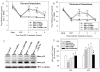
EA treatment at the SP9 acupoint with electrical stimulation (2-Hz frequency) significantly attenuated both thermal (7.8±0.34 sec) and mechanical (41.83±1.22 g) hyperalgesia compared to the no-treatment DNP group (thermal: 5.69±0.39 sec, mechanical: 23.66±1.79 g). EA treatment at the ST36 acupoint with 2-Hz frequency electrical stimulation (33.21±2.51 g) also significantly alleviated mechanical hyperalgesia (Fig. 3A, C).
EA treatment with 2-Hz frequency electrical stimulation at the SP9 acupoint significantly attenuated both thermal (7.8±0.34 sec) and mechanical (41.83±1.22 g) hyperalgesia compared to the 120-Hz frequency treatment groups (thermal: 5.36±0.22 sec, mechanical: 26.86±0.67 g) (Fig. 3B, D). The analgesic effects of EA treatment were at the maximum in the 2 hours after EA treatment.
The combined treatment consisting of EA and a sub-effective dose of MK-801 (0.005 mg) alleviated thermal hyperalgesia (8.94±0.47 sec) compared to the DNP control (5.52±0.27 sec) or 0.005 mg MK-801 treatment (5.55±0.14 sec) groups at 2 hours after treatments (Fig. 4A). In the mechanical stimulation experiments, the EA and sub-effective dose (0.005 mg MK-801) combined treatment (40.75±1.09 g) significantly alleviated hyperalgesia compared to DNP control (24.47±1.04 g) or 0.005 MK-801 treatment (22.14±0.9 g) groups at 2 hours after treatments (Fig. 4B). The analgesic effects of the EA and sub-effective MK-801 dose combination treatment were significantly sustained to 4 hours after treatments and showed as much analgesia as the effective dose (0.01 mg MK-801).
Immunoblots (Fig. 4C) and quantification (Fig. 4D) analysis demonstrated the expression of p35 and its cleavage form p25 at 2 hours after treatment. P35 expression levels in the 0.01 mg MK-801 (94±8.6%) or MK-801 and EA synergetic (101.4±4.59%) treatment groups were increased compared to the DNP group in 0.01 mg MK-801 (94±8.6%) or 0.005 mg MK-801 (101.4±4.59%) treatment groups. p25 expression was significantly increased in DNP (126.22±3.68%), 0.005 mg MK-801 (132.47±7.59%), and 0.01 mg MK-801 (137.47±10.23%) treatment groups. The p25 expression level in the EA and sub-effective dose (0.005 mg MK-801) combined treatment group (105.63±4.2%) was significantly lower than both MK-801 single treatment groups (0.01 mg and 0.005 mg MK-801).
Our results showed the depression of p35 expression until 4 weeks after STZ injection and p35 cleavage to p25 at 4 weeks after STZ injection. Expression of p25, a cleavage product of p35, was significantly higher in 4-week diabetic rats than of 2-week diabetic rats. Also, the thermal paw withdrawal latency in 4-week diabetic rats showed delayed responses to painful thermal stimulation compared with those of 2-week diabetic rats. Some articles have shown hypoalgesia to heat stimulation in the early phase (1~2 weeks after streptozotocin) of streptozotocin-induced diabetic neuropathic pain. However, these studies used mice (Ohsawa et al., 2008) or a different injection method of streptozotocin (Malcangio and Tomlinson, 1998; Morgado and Tavares, 2007). Our results provide further evidence that thermal hypoalgesia in diabetic rat may develop with decreased p35 expression and cleavage of p35 to p25. It could be related to p35 cleavage to p25 at 4 weeks of DNP treatment, and CDK activity and calpain-mediated p35 cleavage in DNP conditions remain to be elucidated.
In rodents, single intra-venous injection of streptozotocin (STZ), a toxin selective for pancreatic β cells, induces a short-term insulin-deficient diabetes and neuropathy. This model follows some of the criteria that define neuropathic pain: hyperalgesia to thermal and mechanical noxious stimuli (Jagodic et al., 2007; Sugimoto et al., 2008; Tsuda et al., 2008). Additionally three days after streptozotocin treatment, there is increased phosphorylation of tau, which is one of the hallmark pathological characteristics of Alzheimer's disease and could be caused by the activation of multiple kinases (Clodfelder-Miller et al., 2006). The conversion of p35 to p25 translated into elevated and mislocalized CDK5 activity, hyperphosphorylation of the neurofilament and tau proteins, cytoskeletal disruption, and neuronal death (Dhavan and Tsai, 2001). It has recently been published that p35 participated in the regulation of nociceptive signaling, and the expression of p35 is up-regulated in nociceptive neurons during peripheral inflammation (Pareek et al., 2006; Utreras et al., 2009). p35 knockout mice (p35-/-) showed delayed responses to painful thermal simulation compared with WT controls, and mice overexpressing p35 were more sensitive to painful thermal stimuli than controls (Pareek and Kulkarni, 2006). The formation of p25 was detected as early as 3 hours after carrageenan-induced peripheral inflammation, and p35 protein levels were apparently increased because of induced inflammation (Pareek et al., 2006).
Acupuncture therapies have become increasingly popular and are prescribed commonly by patients with chronic neurological disorders, including diabetic neuropathic pain (Abuaisha et al., 1998; Hamza et al., 2000; Brunelli and Gorson, 2004). Human case series have demonstrated substantial symptom reduction without added side effects (Goodnick et al., 2000; Ahn et al., 2007). These data suggest that acupuncture is a safe and effective therapy for the long-term management of painful diabetic neuropathy, although its mechanism of action remains speculative. Some animal studies have reported the effect of EA stimulation using a diabetic animal model. Chang et al. suggested that electroacupuncture stimulation at the zhongwan acupoint induces plasma glucose concentration in an insulin-dependent manner (Chang et al., 1999), and the hypoglycemic action of 2-Hz EA at the ST36 acupoint was involved in the serotonin pathway (Chang et al., 2005). Lin et al. reported that chronic electrical stimulation could reduce the functional deficits of diabetic neuropathy (Lin et al., 2005). Our results indicated that EA stimulation increased p35 expression and reduced the cleavage of p35 to p25 in EA and MK-801 synergic treatment more than in an MK-801 monotherapy.
NMDA receptors are involved in persistent pain and play an important role in central sensitization in diabetic neuropathic pain (Karadag et al., 2003; Chen et al., 2009). Although NMDA antagonists are highly effective in reducing neuropathic pain, these agents cause side effects at therapeutic doses including hallucination, dysphoria, and impairment of cognitive function (Sang et al., 2002; Cvrcek, 2008). Therefore, the development of methods with a reduced side effect profile is greatly needed.
The stimulation of NMDA receptors reduces p35 levels via the proteasomal degradation by calpain in primary cortical neurons (Wei et al., 2005; Hosokawa et al., 2006). The N-methyl-d-aspartate (NMDA) receptor agonist glutamate was able to induce p35 cleavage, in a manner dependent on extracellular calcium, and p35 cleavage was prevented by the NMDA antagonist MK-801 (Kerokoski et al., 2004). However, Hosokawa et al. suggested that the cleavage of p35 to p25 was not observed in 5 minute stimulation with NMDA or glutamate, and therefore, the NMDA-induced downregulation of cyclin-dependent kinase 5-p35 had a physiological function in postsynaptic activity (Hosokawa et al., 2006). Many authors have demonstrated that MK-801 reverse diabetes-induced thermal hyperalgesia (Malcangio and Tomlinson, 1998; Begon et al., 2000; Gupta et al., 2003; Karadag et al., 2003; Daulhac et al., 2006). Additionally, a combination of NMDA receptor antagonists with opioids has been shown to be efficient in the treatment of diabetic neuropathic pain (Begon et al., 2002; Bujalska et al., 2008) and other forms of neuropathic pain (Nichols et al., 1997; Malyshkin et al., 2005). It has been well defined that acupuncture induces general analgesic effects in experimental studies, and the participation of various endogenous opioids and their receptors has been widely accepted (Han, 2004; Okada and Kawakita, 2009). Zhang et al. reported that acupuncture at acupoint GB30 and NMDA receptor antagonist synergic treatment attenuates pain threshold in CFA-induced inflammatory hyperalgesia (Zhang et al., 2005). In this study, EA stimulation was applied at acupoint SP9 or ST36 in the diabetic neuropathic pain. But Xing et al. demonstrated that MK-801 blocked the 2-Hz EA-induced spinal long-term depression of C-fiber-evoked potentials in spinal nerve ligation-induced neuropathic pain model (Xing et al., 2007).
Our findings suggest that p35 will be a good target molecule for explaining the mechanism of complicated algesia in diabetic neuropathic pain. Additionally, we evaluated the expression level of p35 or p25 with L4, L5, and L6 segments of the lumbar spinal cord. P35 expression levels in the MK-801 and EA synergetic treatment groups were increased compared to the DNP group or 0.005 mg MK-801 treatment groups. The p25 expression level in the EA and sub-effective dose combined treatment group was significantly lower than both MK-801 single treatment groups (0.01 mg and 0.005 mg MK-801). But immunohistochemistry on spinal dorsal horn of L4, L5, and L6 segments needs further study for evaluating precise role of p35 or p25 in pain transmission. From these findings, the combination of EA with low doses of an NMDA receptor antagonist may provide improved strategies for pain management, thus potentially decreasing drug side effects in patients with persistent pain.
- Abuaisha BB, Costanzi JB, Boulton AJ. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: a long-term study. Diabetes Res Clin Pract 1998;39:115-121.
- Ahn AC, Bennani T, Freeman R, Hamdy O, Kaptchuk TJ. Two styles of acupuncture for treating painful diabetic neuropathy--a pilot randomised control trial. Acupunct Med 2007;25:11-17.
- Begon S, Pickering G, Eschalier A, Dubray C. Magnesium and MK-801 have a similar effect in two experimental models of neuropathic pain. Brain Res 2000;887:436-439.
- Begon S, Pickering G, Eschalier A, Dubray C. Magnesium increases morphine analgesic effect in different experimental models of pain. Anesthesiology 2002;96:627-632.
- Brunelli B, Gorson KC. The use of complementary and alternative medicines by patients with peripheral neuropathy. J Neurol Sci 2004;218:59-66.
- Bujalska M, Malinowska E, Makulska-Nowak H, Gumułka SW. Magnesium ions and opioid agonist activity in streptozotocin-induced hyperalgesia. Pharmacology 2008;82:180-186.
- Chang SL, Lin JG, Chi TC, Liu IM, Cheng JT. An insulin-dependent hypoglycaemia induced by electroacupuncture at the Zhongwan (CV12) acupoint in diabetic rats. Diabetologia 1999;42:250-255.
- Chang SL, Tsai CC, Lin JG, Hsieh CL, Lin RT, Cheng JT. Involvement of serotonin in the hypoglycemic response to 2 Hz electroacupuncture of zusanli acupoint (ST36) in rats. Neurosci Lett 2005;379:69-73.
- Chen SR, Samoriski G, Pan HL. Antinociceptive effects of chronic administration of uncompetitive NMDA receptor antagonists in a rat model of diabetic neuropathic pain. Neuropharmacology 2009;57:121-126.
- Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes 2006;55:3320-3325.
- Cvrcek P. Side effects of ketamine in the long-term treatment of neuropathic pain. Pain Med 2008;9:253-257.
- Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol 2006;70:1246-1254.
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol 2001;2:749-759.
- Goodnick PJ, Breakstone K, Wen XL, Kumar A. Acupuncture and neuropathy. Am J Psychiatry 2000;157:1342-1343.
- Grzeda E, Wiśniewska RJ. Differentiations of the effect of NMDA on the spatial learning of rats with 4 and 12 week diabetes mellitus. Acta Neurobiol Exp (Wars) 2008;68:398-406.
- Gupta M, Singh J, Sood S, Arora B. Mechanism of antinociceptive effect of nimodipine in experimental diabetic neuropathic pain. Methods Find Exp Clin Pharmacol 2003;25:49-52.
- Hamza MA, White PF, Craig WF, Ghoname ES, Ahmed HE, Proctor TJ, Noe CE, Vakharia AS, Gajraj N. Percutaneous electrical nerve stimulation: a novel analgesic therapy for diabetic neuropathic pain. Diabetes Care 2000;23:365-370.
- Han JS. Acupuncture and endorphins. Neurosci Lett 2004;361:258-261.
- Hosokawa T, Saito T, Asada A, Ohshima T, Itakura M, Takahashi M, Fukunaga K, Hisanaga S. Enhanced activation of Ca2+/calmodulin-dependent protein kinase II upon downregulation of cyclin-dependent kinase 5-p35. J Neurosci Res 2006;84:747-754.
- Jagodic MM, Pathirathna S, Nelson MT, Mancuso S, Joksovic PM, Rosenberg ER, Bayliss DA, Jevtovic-Todorovic V, Todorovic SM. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J Neurosci 2007;27:3305-3316.
- Karadag HC, Ulugol A, Tamer M, Ipci Y, Dokmeci I. Systemic agmatine attenuates tactile allodynia in two experimental neuropathic pain models in rats. Neurosci Lett 2003;339:88-90.
- Kerokoski P, Suuronen T, Salminen A, Soininen H, Pirttilä T. Both N-methyl-D-aspartate (NMDA) and non-NMDA receptors mediate glutamate-induced cleavage of the cyclin-dependent kinase 5 (cdk5) activator p35 in cultured rat hippocampal neurons. Neurosci Lett 2004;368:181-185.
- Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkarni AB, Brady RO, Pant HC. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci U S A 2001;98:12742-12747.
- Lin CC, Chen MC, Yu SN, Ju MS. Chronic electrical stimulation of four acupuncture points on rat diabetic neuropathy. Conf Proc IEEE Eng Med Biol Soc 2005;4:4271-4274.
- Malcangio M, Tomlinson DR. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain 1998;76:151-157.
- Malyshkin AA, Medvedev IO, Danysz W, Bespalov AY. Anti-allodynic interactions between NMDA receptor channel blockers and morphine or clonidine in neuropathic rats. Eur J Pharmacol 2005;519:80-85.
- Morgado C, Tavares I. C-fos expression at the spinal dorsal horn of streptozotocin-induced diabetic rats. Diabetes Metab Res Rev 2007;23:644-652.
- Nichols ML, Lopez Y, Ossipov MH, Bian D, Porreca F. Enhancement of the antiallodynic and antinociceptive efficacy of spinal morphine by antisera to dynorphin A (1-13) or MK-801 in a nerve-ligation model of peripheral neuropathy. Pain 1997;69:317-322.
- Ohsawa M, Miyata S, Carlsson A, Kamei J. Preventive effect of acetyl-L-carnitine on the thermal hypoalgesia in streptozotocin-induced diabetic mice. Eur J Pharmacol 2008;588:213-216.
- Okada K, Kawakita K. Analgesic action of acupuncture and moxibustion: a review of unique approaches in Japan. Evid Based Complement Alternat Med 2009;6:11-17.
- Pareek TK, Keller J, Kesavapany S, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB. Cyclin-dependent kinase 5 activity regulates pain signaling. Proc Natl Acad Sci U S A 2006;103:791-796.
- Pareek TK, Kulkarni AB. Cdk5: a new player in pain signaling. Cell Cycle 2006;5:585-588.
- Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg 2003;97:1108-1116.
- Sang CN, Booher S, Gilron I, Parada S, Max MB. Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: efficacy and dose-response trials. Anesthesiology 2002;96:1053-1061.
- Sharma P, Veeranna, Sharma M, Amin ND, Sihag RK, Grant P, Ahn N, Kulkarni AB, Pant HC. Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J Biol Chem 2002;277:528-534.
- Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain 1999;83:389-400.
- Sugimoto K, Rashid IB, Shoji M, Suda T, Yasujima M. Early changes in insulin receptor signaling and pain sensation in streptozotocin-induced diabetic neuropathy in rats. J Pain 2008;9:237-245.
- Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-regulated protein kinase signaling. Glia 2008;56:378-386.
- Utreras E, Futatsugi A, Rudrabhatla P, Keller J, Iadarola MJ, Pant HC, Kulkarni AB. Tumor necrosis factor-alpha regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J Biol Chem 2009;284:2275-2284.
- Wei FY, Tomizawa K, Ohshima T, Asada A, Saito T, Nguyen C, Bibb JA, Ishiguro K, Kulkarni AB, Pant HC, Mikoshiba K, Matsui H, Hisanaga S. Control of cyclin-dependent kinase 5 (Cdk5) activity by glutamatergic regulation of p35 stability. J Neurochem 2005;93:502-512.
- Xing GG, Liu FY, Qu XX, Han JS, Wan Y. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol 2007;208:323-332.
- Yang YR, He Y, Zhang Y, Li Y, Li Y, Han Y, Zhu H, Wang Y. Activation of cyclin-dependent kinase 5 (Cdk5) in primary sensory and dorsal horn neurons by peripheral inflammation contributes to heat hyperalgesia. Pain 2007;127:109-120.
- Zhang RX, Wang L, Wang X, Ren K, Berman BM, Lao L. Electroacupuncture combined with MK-801 prolongs anti-hyperalgesia in rats with peripheral inflammation. Pharmacol Biochem Behav 2005;81:146-151.