Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2013; 22(1): 45-50
Published online March 30, 2013
https://doi.org/10.5607/en.2013.22.1.45
© The Korean Society for Brain and Neural Sciences
Effects of Serotonin on Erythropoietin Expression in Mouse Hippocampus
Miyeon Choi and Hyeon Son*
Department of Biochemistry and Molecular Biology, Hanyang University College of Medicine, Seoul 133-791, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-2220-0626, FAX: 82-2-2220-2422
e-mail: hyeonson@hanyang.ac.kr
Serotonin (5-hydroxytryptamine, 5-HT), a monoamine neurotransmitter, regulates neurological functions such as mood, sleep, and appetite. Erythropoietin (EPO) is well known for erythropoiesis but has recently emerged as a therapeutic agent in brain diseases. However, the mechanisms that induce EPO in the brain remain unclear. The present study was undertaken to investigate whether the effects of 5-HT involve EPO in murine hippocampal neurons. 5-HT produced a significant increase in neuronal differentiation of hippocampal neural progenitor cells. Expression of erythropoietin was increased in 5-HT-treated cells as well. The actions of 5-HT and EPO appeared to be similar in neurite outgrowth and spine formation. In addition, we show that hippocampal expression of EPO was decreased by chronic unpredictable stress (CUS) and that antidepressant treatment to maintain 5-HT concentration in synaptic cleft reversed this effect. In conclusion, actions of antidepressants might involve EPO induction in the brain.
Keywords: erythropoietin, depression, hippocampus
Serotonin (5-HT) is a monoamine neurotransmitter in the central nervous system (CNS) [1]. The serotonergic neurons of the CNS regulate mood, sleep and cognitive functions including memory and learning [2]. 5-HT also plays critical roles in brain development by regulating neurite outgrowth and cell survival [3]. In patients with depression, the level of 5-HT is reduced in the brain, especially hippocampus [4, 5]. It has been suggested that neural atrophy and reduced neurogenesis are key factors in clinical development of depression. The results are supported by the evidence that brain derived neurotrophic factor (BDNF), which plays a pivotal role in neurite outgrowth and neuronal survival, is involved in the antidepressant actions [6]. Numerous studies have revealed that 5-HT increases neurogenesis in the rat brain [7, 8]. Selective serotonin reuptake inhibitors (SSRIs) increase extracellular concentration of 5-HT as inhibitor of the 5-HT reuptake [9]. Among SSRIs, fluoxetine is most widely used to treat depression and stimulates strong neurogenesis in hippocampus [10] where it generates neurons continuously throughout life [11].
EPO was originally described as regulating the synthesis of red blood cells but has recently been suggested as a therapeutic agent in brain disease [12, 13]. A recent study shows that EPO and EPOR are expressed in the central nervous system and that EPO is produced by neurons [14]. EPOR is also expressed in murine hippocampus and cortex [15]. EPO, through binding to its receptor, protects neuronal apoptosis, and increases the expression of BDNF in hippocampus [16]. EPO exerts neuroprotection in neurodegenerative animal model [17]. The neuroprotective effects of EPO are exerted by increasing the level of BDNF and neurogenesis in hippocampus [16]. From the previous studies, it is speculated that there might be a link between actions of EPO and 5-HT in the brain.
We hypothesized that some effect of 5-HT could be mediated by production of EPO. Thus, we investigated whether fluoxetine that maintains concentration of 5-HT in extracellular space as a SSRI, increases EPO in hippocampus, thereby mediating antidepressant efficacy.
MATERIALS AND METHODS
Hippocampi were dissected from embryonic day 14 (E14) mouse embryos into HBSS without calcium or magnesium. Cells were plated on 10-cm dishes coated with 15 µg/ml poly-L-ornithine and 1 µg/ml fibronectin (Invitrogen, Carlsbad, CA, USA) at 2.5×104 cells/cm2 in N2 medium and incubated at 37℃ in 95% air 5% CO2. Basic fibroblast growth factor (bFGF, 20 µg/ml, R&D system, Minneapolis, MN, USA) and epidermal growth factor (EGF, 20 µg/ml, R&D system, Minneapolis, MN, USA) were added daily for 2-3 days in order to expand the population of proliferative progenitors, and the medium was changed every 2 days at the time of bFGF and EGF addition. For high-density cultures, the cells on 80% were subcultured once in N2 medium in the presence of 6 × 104 cells/cm2 in a 24 well plate and these subcultured cells were designated as "passaged once (P1)". The neural progenitors were induced to differentiate by withdrawing growth factors (bFGF, EGF) and kept in a differentiation medium (neurobasal medium add to B27) for 3-5 days. The medium was changed every 3 days. VPA (0.5 µM), 5-HT (10 µM), EPO (10 µg/ml) was treated on 3-5 days following induction of differentiation.
Total RNA from cells was prepared by Trizol reagent (Life technologies Inc., Rockville, MD, USA). For reverse transcription (total reaction volume of 20 µl), 1 µg total RNA was reversely transcribed into cDNA at 70℃ for 10 minutes with a mixture of Oligo (dT) 12-18 primer (Bioneer, Daejeon, South Korea) in 10 µl DEPC-treated water. The reaction solution was mixed with Improm-II 5X buffer (Promega, Madison, WI, USA), 10 mM dNTP (Enzynomics, Daejeon, South Korea), RNAsin (Promega, Madison, WI, USA) at 42℃ for 60 minutes. For quantitative analysis, real-time PCR using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) was performed with iCycler (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions.
The following PCR primers were employed:
EPO sense, 5'-ACT CCG AAC ACT CAC AGT GGA TAC-3', antisense, 5'-GAT TCT GAG GCT CTT CTT CTC TGG-3'.
Protein extracts were mixed with sample loading buffer (62.5 mM Tris-HCL pH 6.8, 2% sodium dodecyl sulfate, 10% glycerol, 50 mM dithiothreitol, 0.1% bromophenol blue) and boiled for denaturation. The samples were then electrophresed on 10% polyacrylamide gels and transferred onto nitrocellulose membrane filters. Blots were blocked with 5% non-fat milk in Tris-buffered saline with Tween-20 (TTBS) for 1 h, incubated for overnight at 4℃ with either rabbit polyclonal EPO (1:500, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA).
Cells were fixed in 4% paraformaldehyde/0.15% picric acid in PBS for 20 minutes. The following primary antibody was used. β-tubulin type III (Tuj1) monoclonal at 1:500 (Covance, Emeryville, CA, USA), microtubule-associated protein-2 (MAP2) monoclonal at 1:500 (Sigma-Aldrich, Saint Louis, MO, USA) were used for detecting. For detection of primary antibodies, anti-mouse IgG (Cell Signaling Technology, Beverly, MA, USA) were used according to the specification of the manufacturer. Cells were mounted in the vectashield mounting medium for fluorescence and photographed with a fluorescent microscope (Nikon, Tokyo, Japan).
Images were acquired through Z-stacks, which typically consists of 10 scans at high zoom at 1-µm steps in the z axis. Each MAP2 (+) neuron was definitely distinguishable from other cells. For an analysis of Spine density, we focused on first-order dendrite from cells. For each cell, 3 dendritic segments were used for a spine analysis. Spine counting was conducted with 60X objective using the Delta Vision Images (Applied Precision, Seattle, USA). The number of spines was counted in a 10 µm segment. For an analysis of dendritic arborization, the Z-trace feature was used to measure the three-dimensional length of the dendritic arbor. With each cell, first-order dendrites arising from the cell bodies were separately traced. They were measured by Image J.
Two-month-old male C57BL/6 mice were housed in a 12-h light/dark cycle animal facility. Mice were daily injected intraperitoneally with saline or fluoxetine (5 mg/kg) for 14 days.
Mice were implanted with a guide cannula into the lateral ventricles (coordinates±0.9 mm (anterior/posterior), ±0.34 mm (lateral), and ±2.15 mm (ventral) relative to the bregma). After a 7 day recovery period, 5-HT (1 µM, 1 µl) was delivered at the rate of 0.25 µl/min with an injection cannula protruding 0.5 mm beyond the guide cannula. The injection cannula stayed in the guide cannula for 10 min after infusion.
Chronic unpredictable stress (CUS) is an experimental procedure in which animals are exposed to a variable sequence of mild and unpredictable stressors. The chronic unpredictable stress was performed similar to published protocol [18]. These sequences of stressors were reported reduction of peripheral brain-derived neurotrophic factor (BDNF) (Table 1) [18]. We used the C57BL/6 mice. Mice were group housed (3-4 mice/cage) and were applied to different stressors per day for 14 days.
All data were measured by Image J software and were expressed as mean±SEM. Statistical significance was determined by Student's
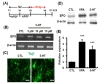
To examine the effect of 5-HT on neuronal differentiation, hippocampal neuronal progenitor cells were incubated with 5-HT in the absence of bFGF for 4 days (Fig. 1A). EPO mRNA expression was increased by 5-HT ranging 5 to 15 µM by RT-PCR (Fig. 1B). Therefore, we used 5-HT at 10 µM concentration
To determine whether EPO is induced upon 5-HT
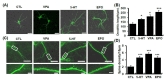
We previously reported that EPO and VPA enhance neurite outgrowth, respectively [19]. To examine whether 5-HT has similar effects, neurite outgrowth was quantified by measuring the lengths of branches extending from MAP2(+) cell soma. The dendritic lengths of MAP2(+) neurons were significantly increased by 5-HT treatment (Fig. 3A). Quantitative analysis revealed that treatment with 5-HT increased the lengths of dendrites comparable to those in the EPO- or VPA-treated cells (Fig. 3B: in µm, CTL, 147.19±19.03; VPA 200.94±24.31, 5-HT 206.65±7.62, EPO 241.25±13.92, *p<0.05, ***p<0.001). To test if 5-HT can also promote the spine formation, we examined spine number in cells treated with 5-HT (Fig. 3C). Treatment cells with 5-HT significantly increased the number of spine in MAP2(+) cells, similarily to the effects of EPO or VPA (Fig. 3D: CTL, 2.28±0.21; VPA, 3.65±0.43; 5-HT, 3.54±0.15; EPO, 3.85±0.39, ***p<0.01) (Fig. 4D). Taken together, these results suggest that 5-HT and EPO enhance the neurite outgrowth and the spine formation during the neuronal differentiation.
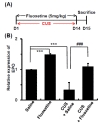
To investigate if the systemic effects of fluoxetine involve EPO, we used a chronic unpredictable stress (CUS) model, a putative animal model of depression. If antidepressant action of fluoxetine involves EPO, the level of EPO expression might be decreased and fluoxetine treatment should recover the EPO expression in CUS animals. Chronic fluoxetine treatment alone had a significant effect on neuritin expression in non-stressed animals (Fig. 4B: ***p<0.001). EPO mRNA levels are significantly decreased in the hippocampus of CUS animals (Fig. 4B: ***p<0.001). In contrast, chronic administration (2 wk, initiated at day 1 of CUS) of the 5-HT selective reuptake inhibitor, fluoxetine, significantly reversed the effects of CUS exposure in the hippocampus (Fig. 4B: fluoxetine, 1.49±0.02; CUS, 0.34±0.23; CUS+FLX, 1.10±0.09, ***p<0.001, ###p<0.001).
Our present study demonstrates that EPO is induced by 5-HT and fluoxetine recovers the downregulation of EPO in the hippocampus of CUS animals. Our results suggest that EPO might act as a downstream molecule in 5-HT signaling pathways in the hippocampus. EPO induction upon 5-HT pulse might contribute to the EPO-mediated cell proliferation and neurogenesis in hippocampus, as previously reported [19]. However, the molecular mechanisms underlying 5-HT induction of EPO need further investigations.
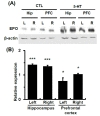
Prefrontal cortex and hippocampus are involved in working memory [21]. In patients with major depression, lower volume of hippocampus and prefrontal cortex has been found [22]. Injection of 5-HT into the lateral ventricles produces EPO induction in the hippocampus and prefrontal cortex with a greater extent in the hippocampus, suggesting that hippocampus might be a primary target area of EPO in the brian.
Previous results showed that neuronal outgrowth is decreased by the long term depression (LTP) of hippocampus [23] and stress [24]. In the present study, we showed that EPO increases the dendrite length and the spine density, consistent with previous results showing EPO stimulates the neurite outgrowth in polarizing hippocampal neurons [25].
Chronic unpredictable stress (CUS) is an well-documented animal model for depression [26].
- Green AR. Neuropharmacology of 5-hydroxytryptamine. Br J Pharmacol 2006;147:S145-S152.
- Zamani A, Qu Z. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS Lett 2012;586:2360-2365.
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 2003;4:1002-1012.
- Coppen A. The biochemistry of affective disorders. Br J Psychiatry 1967;113:1237-1264.
- Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 2001;25:836-844.
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301:805-809.
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience 1999;89:999-1002.
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology 1999;21:46S-51S.
- Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J Neurosci 2004;24:5420-5426.
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20:9104-9110.
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning?. Hippocampus 2006;16:216-224.
- Mohamed HE, El-Swefy SE, Mohamed RH, Ghanim AM. Effect of erythropoietin therapy on the progression of cisplatin induced renal injury in rats. Exp Toxicol Pathol 2013;65:197-203.
- Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol 2007;64:159-171.
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci 2005;6:484-494.
- Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 1997;76:105-116.
- Viviani B, Bartesaghi S, Corsini E, Villa P, Ghezzi P, Garau A, Galli CL, Marinovich M. Erythropoietin protects primary hippocampal neurons increasing the expression of brain-derived neurotrophic factor. J Neurochem 2005;93:412-421.
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A 2000;97:10526-10531.
- Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 2010;35:2378-2391.
- Oh DH, Lee IY, Choi M, Kim SH, Son H. Comparison of neurite outgrowth induced by erythropoietin (EPO) and carbamylated erythropoietin (CEPO) in hippocampal neural progenitor cells. Korean J Physiol Pharmacol 2012;16:281-285.
- Yu IT, Park JY, Kim SH, Lee JS, Kim YS, Son H. Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology 2009;56:473-480.
- Izaki Y, Takita M, Akema T. Specific role of the posterior dorsal hippocampus-prefrontal cortex in short-term working memory. Eur J Neurosci 2008;27:3029-3034.
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jäger M, Leinsinger G, Hahn K, Möller HJ. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry 2002;51:708-714.
- Sdrulla AD, Linden DJ. Double dissociation between long-term depression and dendritic spine morphology in cerebellar Purkinje cells. Nat Neurosci 2007;10:546-548.
- Fricker AD, Rios C, Devi LA, Gomes I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res Mol Brain Res 2005;138:228-235.
- Ransome MI, Turnley AM. Erythropoietin promotes axonal growth in a model of neuronal polarization. Mol Cell Neurosci 2008;38:537-547.
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev 1981;5:247-251.