Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2015; 24(2): 126-132
Published online June 30, 2015
https://doi.org/10.5607/en.2015.24.2.126
© The Korean Society for Brain and Neural Sciences
Aberrant Thalamocortical Synchrony Associated with Behavioral Manifestations in Git1−/− Mice
Won Mah1,2*
1Department of Anatomy and Neurobiology, School of Dentistry, Kyungpook National University, Daegu 700-412, 2Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 305-701, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-53-660-6861, FAX: 82-53-426-7731
e-mail: Wonmah@knu.ac.kr
Cross-talk between the thalamus and cortex has been implicated in attention but its pathogenic role in attention-deficit/hyperactivity disorder (ADHD) remains unknown. Here, I demonstrate that
Keywords: ADHD, GIT1, Thalamic oscillation, Coherence
INTRODUCTION
Among overwhelming amount of sensory signals from all sensory organs, brain selectively processes relevant sensory information at the expense of others. This process of allocating limited processing resources to relevant information is referred to as attention, which has been the focus of various studies because of its importance in human cognition. Thalamus is a brain region that relays sensory and motor information to the cortex and regulates cognitive processes including consciousness, attention, wakefulness, and sleep [1,2,3,4,5,6,7]. Cross-talk between the thalamus and the cortex has been implicated in attention since all sensory information is relayed to the cortex via thalamus [8].
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent psychiatric disorder that affects 5~10% of school-age children worldwide and frequently persists into adulthood [9]. Because of its high prevalence and lifelong impairment, massive researches have been conducted to delineate neural correlates of the disorder. Though several lines of evidences imply the association between abnormalities in thalamus and ADHD [10,11,12], the pathogenic role of the thalamus in ADHD is still elusive.
GIT1 (G protein-coupled receptor kinase-interacting protein-1) is a multifunctional signaling adaptor associated with ADHD [13].
Here, I report that the thalamus of
MATERIALS AND METHODS
Amphetamine (U.S. Pharmacopia) and ethosuximide (Tokyo Chemical Industry) were dissolved in 0.9% saline and distilled water to final concentrations of 1.2 g/L and 60 g/L, respectively. Solutions for injection were filtered with Minisart filter (0.2 µm; Sartorius Stedim Biotech). WT and
Mice were anesthetized by ketamine (Yuhan Corporation). For depth recording, a parylene-coated tungsten electrode (0.005 in, 2 MΩ, A-M Systems, Inc.) was implanted into the ventrobasal or mediodorsal nucleus of the thalamus with grounding electrodes over the cerebellum. LFP recordings were performed 1 week after the implantation. LFP activities (sampled at 200 Hz) of freely moving mice were recorded for 1 h using the NACGather program (Theta Burst Corp.). Amphetamine was delivered intraperitoneally to mice 20 min after basal LFP recording. LFP activity was recorded for 1 h briefly after the injection. LFP recordings were analyzed by Matlab, using EEGLAB and custom-written coding.
The size of the open field box was 40×40×40 cm, and the center zone line was located 6.5 cm apart from the edge. Mice were placed in the center of the chamber in the beginning of the assay, and spontaneous locomotion activity was observed and recorded for 60 min in an open field chamber, briefly after injection. The results were analyzed by Ethovision 3.1 program (Noldus). The total distance moved was obtained by summing the movements made during 10~60 min. All behavioral assays were performed in a blind manner.
The apparatus used in the open field tests was used for object recognition tests. During the sample phase, mice were allowed to explore two identical objects for 10 min. Objects were put in the center of the chamber, and mice were first put in the chamber facing the wall. Exploration time for each object was measured. In the test phase, performed 24 h later, one of the two objects was replaced with a new one, and exploration time for each object was measured. All objects were pre-tested to confirm that there was no difference in object preference using C57BL/6 wild-type mice and C57BL/6-129/SV/Jae hybrid wild-type mice.
As previous studies have identified close connection between ventrobasal thalamus and prefrontal cortex during attention task [15,16], I hypothesized that abnormal theta oscillation in the PFC of
Thus, I examined the functional properties of the ventrobasal thalamus of
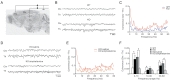
Abnormal theta rhythms in the human cortex are thought to be coupled with those in the thalamus through the thalamocortical pathway, a process that has been termed thalamocortical dysrhythmia (TCD) [17]. To this end, I simultaneously measured cortical EEG rhythms and thalamic activities (LFP) in WT and
Increased coherence between the
Inhibitory inputs into the thalamus facilitate hyperpolarization of thalamic neurons, which is required for the recovery of T-type calcium channels from inactivation and low-threshold spikes generation. The latter is a neuronal burst activity closely related to thalamic rhythms that are conveyed to other brain regions, including cortex [19]. Ethosuximide, which is mainly used as an antiepileptic agent, is a well-known T-type calcium channel blocker and suppressant of thalamic bursts [20]. Here, I tested whether ethosuximide could suppress 3-Hz rhythms in the
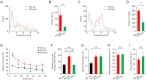
Similar to the effects of amphetamine, ethosuximide normalized 3-Hz thalamic rhythms in
Unlike the reported rescue of novel-object recognition memory in
I herein provide evidence suggesting that enhanced 3-Hz rhythms in the thalamus may contribute to enhanced cortical theta EEG rhythms and hyperactivity in
The 3-Hz rhythms in the
The circuit-level mechanism for enhanced 3-Hz rhythms in the thalamus of
Another source of inhibitory inputs to the thalamus comes from endopeduncular nucleus (analogous to the internal globus pallidus in primates), which is negatively regulated by the globus pallidus directly or indirectly via subthalamic nucleus [26,27]. Inhibitory projections from the endopeduncular nucleus to the thalamus significantly influence firing rates and rhythmic activities of the ventrolateral and intralaminar thalamic nuclei [26]. Globus pallidus also modulates thalamus via its direct and inhibitory modulation of TRN neurons [28]. Amphetamine may indirectly regulate GABAergic neurons, as psychostimulants suppress the stimulatory action of the locus coeruleus onto the TRN [29].
Finally, I found that ethosuximide failed to restore novel object recognition while correcting hyperactivity in
In summary, my data indicate that 3-Hz thalamic rhythms are associated with cortical theta EEG rhythms and hyperactivity in


- Llinás RR, Paré D. Of dreaming and wakefulness. Neuroscience 1991;44:521-535.
- Llinás R, Ribary U. Consciousness and the brain. The thalamocortical dialogue in health and disease. Ann N Y Acad Sci 2001;929:166-175.
- Kinomura S, Larsson J, Gulyás B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 1996;271:512-515.
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci 2001;24:122-126.
- Swadlow HA, Gusev AG. The impact of 'bursting' thalamic impulses at a neocortical synapse. Nat Neurosci 2001;4:402-408.
- Alitto HJ, Usrey WM. Corticothalamic feedback and sensory processing. Curr Opin Neurobiol 2003;13:440-445.
- Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol 2006;95:3297-3308.
- Faw B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: a tutorial review. Conscious Cogn 2003;12:83-139.
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition?. World Psychiatry 2003;2:104-113.
- Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, Sanchez-Pena J, Miller AM, Chakravarty MM, Klahr K, Durkin K, Greenhill LL, Peterson BS. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry 2010;167:397-408.
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry 2006;47:1051-1062.
- Shaw P. The shape of things to come in attention deficit hyperactivity disorder. Am J Psychiatry 2010;167:363-365.
- Won H, Mah W, Kim E, Kim JW, Hahm EK, Kim MH, Cho S, Kim J, Jang H, Cho SC, Kim BN, Shin MS, Seo J, Jeong J, Choi SY, Kim D, Kang C, Kim E. GIT1 is associated with ADHD in humans and ADHD-like behaviors in mice. Nat Med 2011;17:566-572.
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol 2003;114:171-183.
- Cao XH, Wang DH, Bai J, Zhou SC, Zhou YD. Prefrontal modulation of tactile responses in the ventrobasal thalamus of rats. Neurosci Lett 2008;435:152-157.
- Burton H, Abend NS, MacLeod AM, Sinclair RJ, Snyder AZ, Raichle ME. Tactile attention tasks enhance activation in somatosensory regions of parietal cortex: a positron emission tomography study. Cereb Cortex 1999;9:662-674.
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A 1999;96:15222-15227.
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 2007;30:317-324.
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 1997;20:185-215.
- Coulter DA, Huguenard JR, Prince DA. Specific petit mal anticonvulsants reduce calcium currents in thalamic neurons. Neurosci Lett 1989;98:74-78.
- Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol 1989;25:582-593.
- Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J Neurosci 1994;14:5485-5502.
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev 2004;46:1-31.
- Avanzini G, Panzica F, de Curtis M. The role of the thalamus in vigilance and epileptogenic mechanisms. Clin Neurophysiol 2000;111:S19-S26.
- Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci 2005;28:317-324.
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 1989;12:366-375.
- Kita H. Globus pallidus external segment. Prog Brain Res 2007;160:111-133.
- Kayahara T, Nakano K. The globus pallidus sends axons to the thalamic reticular nucleus neurons projecting to the centromedian nucleus of the thalamus: a light and electron microscope study in the cat. Brain Res Bull 1998;45:623-630.
- Rowe DL, Robinson PA, Gordon E. Stimulant drug action in attention deficit hyperactivity disorder (ADHD): inference of neurophysiological mechanisms via quantitative modelling. Clin Neurophysiol 2005;116:324-335.



