Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2015; 24(3): 226-234
Published online September 30, 2015
https://doi.org/10.5607/en.2015.24.3.226
© The Korean Society for Brain and Neural Sciences
Modulation of SOD1 Subcellular Localization by Transfection with Wild- or Mutant-type SOD1 in Primary Neuron and Astrocyte Cultures from ALS Mice
Do-Yeon Lee1#, Gye Sun Jeon1,2#, Yu-mi Shim1, Seung-Yong Seong3, Kwang-Woo Lee1 and Jung-Joon Sung1*
1Department of Neurology, 2Biomedical Research Institute, Seoul National University Hospital College of Medicine, 3Wide River Institute of Immunology, Department of Microbiology and Immunology, Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 03080, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-2072-1015, FAX: 82-2-3672-7553
e-mail: jjsaint@snu.ac.kr
#These authors contributed equally to this work.
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurological disorder characterized by selective degeneration of motor neurons. Mutant superoxide dismutase 1 (SOD1) is often found as aggregates in the cytoplasm in motor neurons of various mouse models and familial ALS patients. The interplay between motor neurons and astrocytes is crucial for disease outcome, but the mechanisms underlying this phenomenon remain unknown. In this study, we investigated whether transient transfection with wild-type and mutant-type SOD1 may lead to amplification of mutant SOD1-mediated toxicity in cortical neurons and astrocytes derived from wild-type and mutant-type (human G93A-SOD1) mice. In transgenic mice expressing either wild- or mutant-type SOD1, we found that green fluorescent protein (GFP)-wtSOD1 was present in the cytoplasm and nuclei of wild-type cortical neurons and astrocytes, whereas GFP-mutant SOD1 was mainly cytoplasmic in wild- and mutant-type cortical neurons and astrocytes. These findings indicate that intracellular propagation of misfolding of GFP-wt or mtSOD1 are possible mediators of toxic processes involved in initiating mislocalization and aggregation. Here, we provide evidence that cytoplasmic aggregates induce apoptosis in G93A-SOD1 mouse cortical neurons and astrocytes and that the toxicity of mutant SOD1 in astrocytes is similar to the pathological effects of ALS on neurons
Keywords: Amyotrophic lateral sclerosis, cortical astrocyte, cortical neuron, G93A SOD1, mislocalization, apoptosis
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) results from progressive loss of upper and lower motor neurons and denervation atrophy of skeletal muscles [1,2]. However, the mechanisms underlying this selective degeneration remain unclear. Many studies have shown that ALS is at least partially a non-cell-autonomous disease and that cells such as astrocytes expressing mutant hSOD1 contribute to the pathogenesis of ALS [3]. In
A pathologic hallmark of fALS is aggregation of mutant SOD1 [6]. Mutant SOD1 forms proteinaceous inclusions in the tissues of ALS patients, in transgenic mice, and in cultured cells [6,7,8]. In ALS, spinal cord proteins are subject to oxidative damage, and motor neurons are particularly vulnerable to oxidative stress. Another prominent feature of the pathology of ALS is the generation and migration of new cells, particularly astrocytes, within and around damaged regions [9]. The interplay between motor neurons and glial cells is important in the clinical progression of both familial and sporadic motor neuron diseases. ALS-associated mutations in SOD1, both missense and premature termination, include misfolding and aggregation of the protein [10]. However, the molecular features of the mutant SOD1 responsible for initiating disease have yet to be defined. Recent studies have they brought attention to this question role of WT SOD1 as a modulator of disease initiation. In mice expressing a mutant protein that is easily distinguished from WT SOD1, such as the G85R, T116X, and L126Z mutants, it has been possible to determine that earlier disease onset is accompanied by the formation of detergent-insoluble aggregates that appear to contain both WT hSOD1 and mutant hSOD1 [11].
MATERIALS AND METHODS
Transgenic mice originally obtained from Jackson Laboratories (Bar Harbor, ME, USA) and expressing a high copy number of mutant human SOD1 with Gly93Ala substitution (TgSOD1 G93A) were bred and maintained on a B6/SJL mice strain. Tail samples were collected from the embryos for genotyping. All procedures were performed in accordance with the Institutional Animal Care and Use Committee (IAUCC) guidelines of Seoul National University for the care and use of laboratory animals.
Cultures were prepared as previously described [13]; briefly, cortical neurons were isolated from pups at E16.5, and the brains were removed and placed on ice in Hank's Balanced Salt Solution (HBSS, Gibco). Twenty-four-well plates were seeded with 1 ml of plating medium (2% B27+1/100 penicillin/streptomycin, Invitrogen; 0.5 mM α-glutamine; neurobasal medium, Gibco). From DIV4, 10% fresh medium was added every 3~5 days. Cultured mouse astrocytes were prepared as described previously [14]. Briefly, cortices of 1-day-old mouse pups were dissected out and digested with trypsin for 10 min at 37℃. Single-cell suspensions were obtained by trituration, and cells were seeded onto poly-d-lysine-coated plates. Cultures were maintained in DMEM/F12 containing 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin. The cells were allowed to proliferate until they reached confluence. At this point, arabinofuranosylcytidine (AraC, 10 µM; Sigma) was added to prevent the growth of oligodendrocytes. These cultures were maintained in complete medium.
Cultured primary cortical neurons and astrocytes were seeded and allowed to attach before transfection with either green fluorescent protein (GFP)-wtSOD1 or mtSOD1 by using the transfection reagent supplied by the manufacturer, according to the instructions provided by the manufacturer (LipofectAMINE 2000 reagent, Invitrogen) for 24 h or 48 h. Transfection was performed in a medium containing 10% FBS but without antibiotics. Cultured neurons and astrocytes treated with the pcN1 vector alone were used as negative controls. Transfection efficiency was tested by using a vector containing the GFP gene.
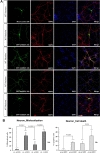
Cells were plated on poly-d-lysine-coated 24-well or 6-well plates (SPL). Astrocytes and neuronal cells (24~48 h after transfection) were fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature and then washed three times in PBS. Immunofluorescence staining and confocal microscopy were used to determine the levels of glial fibrillary acidic protein (GFAP, Sigma) and MAP-2 (NeoMarkers). The specimens were incubated for 1 h with anti-mouse secondary antibody conjugated with Alexa 555 (1:200, Invitrogen) after incubation with primary antibodies. Images were analyzed using a fluorescence microscope (Leica) coupled to a digital camera. Control experiments were performed in the absence of the primary antibody or in the presence of blocking peptide. GFP-fluorescent neurons and astrocytes were visualized with epifluorescent illumination on a Leica microscope. Subcellular localization patterns were documented with a ×20 objective lens; total GFP-positive neurons and astrocytes were counted off-line within 20 randomly chosen fields in at least three independent cultures, and the percentage of mislocalized and aggregated neurons and astrocytes within the total number of GFP-positive cells was calculated. The cell death ratio was estimated by counting nuclear fragmented cell identification with DAPI nuclear stain. The cell death were documented with a ×20 objective lens; total GFP-positive neurons and astrocytes were counted off-line within 20 randomly chosen fields in at least three independent cultures and in duplicate.
Western blot analysis was performed as previously described [15]. Cell lysates (30 µg protein) were analyzed by Western blot using anti-cleaved caspase-3 (Cell Signaling), SOD1 (Abcam), and anti-GFP (Rockland) antibodies. Protein loading was controlled by probing for β-actin (Santa Cruz Biotech) on the same membrane.
Data were analyzed using Prism software (GraphPad Software, San Diego, CA, USA), using either Student's
RESULTS
To investigate whether the intracellular localization of SOD1 is altered by transient transfection with GFP-wt and mtSOD1 in WT or MT neurons, we transfected WT or MT cortical neurons with plasmids encoding either GFP-wt or G93A-SOD1 (mtSOD1), and observed the resulting changes in GFP-wt or mtSOD1 (Fig. 1A). Immunocytochemical analysis was performed on mice embryonic cortical neurons using MAP-2 as a neuronal marker. The data showed that in WT neurons, GFP-wtSOD1 localized to both the cytoplasm and the nucleus; cell death was not observed (Fig. 1A, B). In MT neurons, GFP-wtSOD1 was excluded from the nuclei; slightly increased rates of cell death were observed (Fig. 1A, B). Interestingly, in WT neurons transfected with GFP-mtSOD1, greater mislocalization was observed than in MT neurons transfected with GFP-wtSOD1 (Fig. 1A, B). The results showed that MT neurons transfected with mtSOD1 were more susceptible to mislocalization and cell death; this effect was strongest in MT neurons transfected with GFP-mtSOD1 (Fig. 1A, B). We also analyzed the effect of GFP-wt and mtSOD1, using DAPI staining for detecting cell death. Both WT neurons transfected with GFP-mtSOD1 and MT neurons transfected with GFP-mtSOD1 showed strongly increased mislocalization. However, only MT neurons transfected with GFP-mtSOD1 showed severely increased cell death, compared with WT neurons transfected with GFP-mtSOD1 (Fig. 1A, B). These results indicate that transient transfection with GFP-wtSOD1 can modulate cell death in MT neurons.
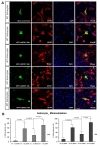
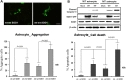
To assess cellular localization against mtSOD1 misfolding and aggregation, we transfected WT or MT astrocytes with plasmids encoding either GFP-wt or mtSOD1, and observed the resulting changes in GFP-wt or mtSOD1 (Fig. 2A). Primary astrocytes were stained with GFAP and DAPI nuclear counterstain. WT astrocytes transfected with GFP-wtSOD1 predominantly colocalized in the cytoplasm and the nucleus, whereas those transfected with GFP-mtSOD1 localized in the cytosol (Fig. 2A). Cytoplasmic mislocalization was examined by detecting GFP-mtSOD1 at 24 h and 48 h post-transfection (Fig. 2B). However, cytoplasmic aggregates were predominantly examined by detecting the presence of GFP-mtSOD1 at 48 h post-transfection (Fig. 3A). We also evaluated the ability of GFP-mtSOD1 to modulate the aggregation of not only MT astrocytes but also WT astrocytes. At 48 h after transfection, we observed significant accumulation of aggregated SOD1 proteins in astrocytes transfected with GFP-mtSOD1. The cytoplasmic localization of aggregates containing mtSOD1 can clearly be seen in the fluorescence images. The aggregate formation ratio was measured by level of hSOD1-EGFP fluorescence in the cytoplasmic area (Fig. 3A). We detected a robust increase in cytoplasmic GFP-mtSOD1 relative to wtSOD1. However, in MT astrocytes transfected with wtSOD1, fewer aggregates were detectable than in WT astrocytes transfected with GFP-mtSOD1 (Fig. 3A). This finding indicates that higher levels of wtSOD1 modulate the generation of these aggregates in MT astrocytes. To estimate cellular apoptosis in response to mtSOD1 mislocalization and aggregation, we observed transfected WT or MT astrocytes. The cell death ratio was estimated by counting apoptosis morphological identification with DAPI nuclear stain. At 48 h after transfection, we observed significant cell death in astrocytes transfected with GFP-mtSOD1. However, in the MT astrocytes transfected with wtSOD1, rates of cell death were lower than in WT astrocytes transfected with GFP-mtSOD1 (Fig. 3B). This finding indicates that higher levels of wtSOD1 modulate the rate of cell death in MT astrocytes. Next, we investigated whether or not cytoplasmic accumulation of mtSOD1 aggregates promotes apoptosis. Compared to WT astrocytes transfected with GFP-wtSOD1, the expression of cleaved-caspase-3 was strongly increased in WT and MT astrocytes transfected with GFP-mtSOD1 (Fig. 3B). In addition, cleaved caspase-3 expression remained detectable in WT astrocytes transfected with GFP-mtSOD1, suggesting that activation of apoptosis is dependent on mtSOD1 (Fig. 3B). Thus, we propose that mutant G93A-SOD1 mediates toxic events in apoptosis, with large detergent-insoluble aggregates, formed of wtSOD1 and transfected mtSOD1, playing roles in the events that initiate disease.
DISCUSSION
In the present study, we provide evidence that the cytoplasmic mislocalization and aggregation of mutant SOD1 induce cell death in wild and G93A-SOD1 mouse primary neurons and astrocytes. These findings indicate that intracellularly propagated GFP-mtSOD1 is a possible mediator of toxic processes and is involved in initiating mislocalization and aggregation. We revealed that levels of mtSOD1 are lower than those of wtSOD1 in the nuclei of both neurons and astrocytes of transgenic mice expressing either human wtSOD1 or mutant G93A-SOD1. This may be because of the formation of insoluble high-MW species of mutant G93A-SOD1 that prevent diffusion of the protein across the nuclear membrane; wtSOD1 can diffuse across the nuclear membrane. Alternatively, nuclear deprivation of mtSOD1 might result from its more rapid rate of turnover in this compartment than wtSOD1. A previous study has shown the possible role of wtSOD1 as a modulator of disease initiation [11]. In addition,
SOD1 activity is generally reduced to approximately 50% of normal levels in patients with SOD1-fALS, owing to the reduced half-life of SOD1 mRNA in the CNS, and the possible effects of SOD1 protein misfolding and aggregation on activity. Our findings indicate that the effect of wtSOD1 on the toxicity of mtSOD1 is influenced by expression of mtSOD1 in both neurons and astrocytes. Using cell culture models of aggregation, we found that wtSOD1 modulates the aggregation of mutant proteins for formation of large detergent-insoluble structures. In MT cells, transfected GFP-wtSOD1 implicated soluble assemblies of wt and mtSOD1 as possible mediators of toxic processes involved in initiating mislocalization and aggregates. In this study, it seemed reasonable to assume that the above mechanism was relevant to both neurons and astrocytes because both cell types show similar effects in fALS. The present results indicate that cortical neurons have selective vulnerability to cell death following transfection with mtSOD1 and that the toxicity of mutant SOD1 on astrocytes is very similar to the pathological conditions of ALS observed in neurons
These findings indicate that soluble assemblies of wt and mtSOD1 are possible mediators of the toxic processes involved in initiating mislocalization and aggregation. Mutant SOD1 is likely interacting with wtSOD1 to produce the augmented toxicity in primary cortical neurons and astrocytes. In addition, these results show that the toxicity of mutant SOD1 in astrocytes mimics the pathological condition of ALS in neurons
Supplementary Material
Supplementary Fig. 1
Cellular and morphological characterization of neurons and astrocytes enriched cultures. (A, B) Immunofluorescent staining for SOD1 of cortical neurons (red) and astrocytes (green) with DAPI for nuclei (blue). (C) Western blot analysis confirmed the presence of the human SOD1 in cortical neurons and astrocytes. Levels of hSOD1 in both mutant cortical neurons and mutant astrocytes identified compared with wild-type.
en-24-226-s001.pdfFigures
References
- Okado-Matsumoto A, Fridovich I. Amyotrophic lateral sclerosis: a proposed mechanism. Proc Natl Acad Sci U S A 2002;99:9010-9014.
- Al-Chalabi A, Leigh PN. Recent advances in amyotrophic lateral sclerosis. Curr Opin Neurol 2000;13:397-405.
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 2009;187:761-772.
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci 2007;10:608-614.
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 2007;10:615-622.
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 1998;281:1851-1854.
- Son M, Cloyd CD, Rothstein JD, Rajendran B, Elliott JL. Aggregate formation in Cu,Zn superoxide dismutase-related proteins. J Biol Chem 2003;278:14331-14336.
- Watanabe M, Dykes-Hoberg M, Culotta VC, Price DL, Wong PC, Rothstein JD. Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol Dis 2001;8:933-941.
- Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estévez AG, Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev 2004;47:263-274.
- Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR. Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet 2003;12:2753-2764.
- Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, Lukas T, Dal Canto MC, O'Halloran TV, Siddique T. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A 2006;103:7142-7147.
- Chattopadhyay M, Durazo A, Sohn SH, Strong CD, Gralla EB, Whitelegge JP, Valentine JS. Initiation and elongation in fibrillation of ALS-linked superoxide dismutase. Proc Natl Acad Sci U S A 2008;105:18663-18668.
- Hilgenberg LG, Smith MA. Preparation of dissociated mouse cortical neuron cultures. J Vis Exp 2007;10:562.
- Jeon GS, Nakamura T, Lee JS, Choi WJ, Ahn SW, Lee KW, Sung JJ, Lipton SA. Potential effect of S-nitrosylated protein disulfide isomerase on mutant SOD1 aggregation and neuronal cell death in amyotrophic lateral sclerosis. Mol Neurobiol 2014;49:796-807.
- Schildge S, Bohrer C, Beck K, Schachtrup C. Isolation and culture of mouse cortical astrocytes. J Vis Exp 2013;19:pii.
- Andrus PK, Fleck TJ, Gurney ME, Hall ED. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem 1998;71:2041-2048.
- Khare SD, Caplow M, Dokholyan NV. The rate and equilibrium constants for a multistep reaction sequence for the aggregation of superoxide dismutase in amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 2004;101:15094-15099.
- Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A. Monomeric Cu,Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J Biol Chem 2004;279:15499-15504.
- Ezzi SA, Urushitani M, Julien JP. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J Neurochem 2007;102:170-178.
- Wilcox KC, Zhou L, Jordon JK, Huang Y, Yu Y, Redler RL, Chen X, Caplow M, Dokholyan NV. Modifications of superoxide dismutase (SOD1) in human erythrocytes: a possible role in amyotrophic lateral sclerosis. J Biol Chem 2009;284:13940-13947.
- Chia R, Tattum MH, Jones S, Collinge J, Fisher EM, Jackson GS. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS One 2010;5:e10627.
- Münch C, O'Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci U S A 2011;108:3548-3553.
- Ravits J, Laurie P, Fan Y, Moore DH. Implications of ALS focality: rostral-caudal distribution of lower motor neuron loss postmortem. Neurology 2007;68:1576-1582.
- Pokrishevsky E, Grad LI, Yousefi M, Wang J, Mackenzie IR, Cashman NR. Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis. PLoS One 2012;7:e35050.



