Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2016; 25(5): 262-268
Published online October 31, 2016
https://doi.org/10.5607/en.2016.25.5.262
© The Korean Society for Brain and Neural Sciences
Systematic Analysis of Translocator Protein 18 kDa (TSPO) Ligands on Toll-like Receptors-mediated Pro-inflammatory Responses in Microglia and Astrocytes
Ji-Won Lee1, Hyeri Nam1 and Seong-Woon Yu1,2*
1Department of Brain and Cognitive Sciences, 2Neurometabolomics Research Center, Daegu Gyeongbuk Institute of Science and Technology (DGIST), Daegu 42988, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-53-785-6113, FAX: 82-53-785-6109
e-mail: yusw@dgist.ac.kr
Translocator protein 18 kDa (TSPO) is a mitochondrial protein highly expressed on reactive microglia and astrocytes, and is considered as a biomarker for neurodegeneration and brain damage, especially neuroinflammation. Toll-like receptors (TLRs) are closely related with inflammatory responses of microglia and astrocytes and these signaling pathways regulate neuroinflammation. Previous reports have identified the anti-inflammatory effects of TSPO ligands, however study of their effects in relation to the TLR signaling was limited. Here, we investigated the effects of five representative TSPO ligands on microglia and astrocytes following activation by various TLR ligands. Our results show that TSPO ligands reduce the pro-inflammatory response elicited by the TLR ligands with more profound effects on microglia than astrocytes.
Keywords: TSPO, TSPO ligands, Toll-like receptors, microglia, astrocyte
INTRODUCTION
Translocator protein 18 kDa (TSPO), previously called peripheral benzodiazepine receptor (PBR), is located in mitochondrial outer membrane [1]. The expression levels of TSPO are increased under various neuroinflammatory conditions in both microglia and astrocytes [2,3]. Many previous studies have demonstrated that the function of TSPO is associated with anti-neuroinflammatory responses in glial cells, while a few studies reported the opposite action of TSPO [4,5,6]. Of note, the pharmacological effects of TSPO ligands were consistently immune suppressive, and therefore, have been explored as potential neurotherapeutic agents in experimental models of Alzheimer's disease (AD), depression, anxiety, multiple sclerosis, brain injuries and neuroinflammation [3,7,8].
Toll-like receptors (TLRs) are the types of pattern-recognition receptors (PRRs) that respond to misfolded proteins, toxins, pathogens, degeneration and traumata [9,10]. Activation of TLRs in neuroimmune cells typically promotes the synthesis and secretion of pro-inflammatory cytokines to extracellular space with implication to various neuroinflammatory diseases [9,10]. However, the stimulation of TLRs can also modulate neuroinflammation and produce anti-inflammatory cytokines [9,11]. Therefore, an increasing amount of evidence has indicated that dysregulation of TLRs and their signaling pathways may play a critical role in various neurodegenerative diseases through proand anti-inflammatory responses in microglia and astrocytes [5,7,10].
Previous studies have reported the anti-inflammatory effects of TSPO ligands in microglia, but those studies on the effects of TSPO ligands and their action mechanisms were mostly limited to lipopolysaccharide (LPS, TLR4 ligand)-stimulated conditions [5,7]. Furthermore, the effects of the TSPO ligands are currently little known in astrocytes [3]. For these reasons, we performed systematical comparative analysis of the anti-inflammatory effects of five widely used TSPO ligands on primary microglia and astrocytes treated with various TLR ligands. Our study shows that five TSPO ligands representing different structural classes reduce the secretion of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and C-C motif chemokine ligand 2 (CCL2) in TLR ligands-activated microglia and astrocytes, albeit with more substantial effects on microglia. Therefore, these results demonstrate that TSPO ligands can be the efficient anti-inflammatory drugs through inhibition of pro-inflammatory responses in both microglia and astrocytes.
MATERIALS AND METHODS
Primary mouse microglia and astrocytes were prepared from C57BL/6J mice and cultured with Dulbecco's Modified Eagle Medium, DMEM (Corning, NY, USA), supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS, Hyclone, Logan, UT, USA) and 1% penicillin-streptomycin (Hyclone). All procedures for the care and use of laboratory animals were approved by the Institutional Animal Care and Use Committee of DGIST. Microglia and astrocytes were obtained from neonatal mice (age 1~3 days) as described previously [12,13]. The neonatal brains were minced and trypsinized, and then plated in a 100-mm dish per 1 brain in DMEM medium containing 10% HI-FBS and 1% penicillin-streptomycin. For isolation of primary microglia, media were replaced with fresh media 4~5 days later, and thereafter half of the media were changed every 2 days with fresh media for 4~8 days. At days 9~12 in vitro, primary microglia were isolated by tapping on the 100-mm dish. For isolation of primary astrocytes, media were replaced with fresh media 3 days later, and the media were replaced every 2 days with fresh media for 7~8 days. At days 10~11 in vitro, primary astrocytes were detached by trypsin-EDTA after removed microglia and oligodendrocyte precursor cells by shacking the dish and tapping.
1-(2-Chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide (PK11195) and 7-chloro-5-(4-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one (Ro5-4864; 4'-chlorodiazepam) were purchased from Sigma-Aldrich (St. Louis, MO, USA). N-Ethyl-7,8-dihydro-7-methyl-8-oxo-2-phenyl-N-(phenylmethyl)-9H-purine-9-acetamide (XBD-173; Empapunil; AC5216), N, N-dihexyl-2-(4-fluorophenyl) indole-3-acetamide (FGIN-1-27) and 6-chloro-2-N-ethyl-4-methyl-4-phenyl-4H-3,1-benzoxazin-2-amine hydrochloride (Etifoxine) were purchased from Tocris Biosciences (Avonmouth, Bristol, United Kingdom). The stock solutions of PK11195 (50 mM) and Ro5-4864 (25 mM) were prepared in absolute ethanol, and those of XBD-173 (100 mM), FGIN-1-27 (100 mM) and Etifoxine (100 mM) in dimethyl sulfoxide. Pam3CSK4 (tripalmitoylated lipopeptide), HKLM (heat-killed preparation of
Primary mouse microglia and astrocytes (3×105 cells/ml) were treated with 50 µM of each TSPO ligand for 1 h prior to stimulation with Pam3CSK4 (100 ng/ml), HKLM (107 cells/ml), Poly (I:C) HMW (10 µg/ml), Poly (I:C) LMW (1 µg/ml), LPS (100 ng/ml), ST-FLA (1 µg/ml), FSL-1 (100 ng/ml), ssRNA (1 µg/ml) and ODN1826 (0.5 µM) for 12 h or 24 h, respectively.
Microglia and astrocytes were fixed by 4% paraformaldehyde for 15 min. After fixation, the cells were rinsed three times with phosphate buffered saline (PBS) and permeabilized with 0.1% Triton X-100 in PBS for 5 min and then blocked with 5% BSA in PBS for 30 min. Cells were reacted with the primary antibody for 2 h at room temperature, and then cells were rinsed three times with PBS and reacted with secondary antibody (goat anti-rabbit Alexa Fluor 488) for 1 h at room temperature. Cell nuclei were stained with Hoechst 33342. The fluorescence images were obtained using Nikon Eclipse 90i fluorescent microscopy.
ELISA kits for the mouse TNF-α, IL-6 and CCL-2 were purchased from R&D systems (Minneapolis, MN, USA) and used to measure mouse TNF-α, IL-6 and CCL2 in the supernatants according to manufacturer's instructions.
After stimulation of TLR ligands, cells were lysed in 1% Triton X-100 lysis buffer (1% Triton X-100, 250 mM sucrose, 20 mM Tris-HCl (pH 7.2), 1 mM EDTA (pH 8.0), 1 mM phenylmethylsulfonyl fluoride, 50 mM NaCl) with 1× protease and phosphatase inhibitors and then cell lysate were separated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane. Membranes were incubated with antibodies specific for TSPO (ab109497, Abcam, Cambridge, UK) or β-actin HRP (sc-47778 HRP, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Bound TSPO antibody was detected by species-specific, horseradish peroxidase-conjugated secondary antibody. Chemiluminescence detection was performed to analyze the protein bands of interest.
After treatment with the 50 µM of TSPO ligands for 24 h, primary mouse microglia and astrocytes were stained with propidium iodide (PI) and Hoechst 33342 (Life Technologies, Grand Island, NY, USA) for 20 min at 37℃. Total cells (Hoechst 33342-positive) and dead cells (PI-positive) were counted under a fluorescence microscope. The percentage of cell death was obtained as follows: Cell death (%)=(PI positive cell number/Hoechst positive cell number)×100.
All data are expressed as the mean±SEM. Statistical analysis was performed with the unpaired two-tailed Student's
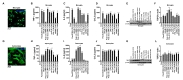
The expression levels of TSPO are highly increased under diverse neuropathological conditions, especially in microglia and astrocytes. However, the regulation of TSPO expression by pro-inflammatory signaling pathways was not fully characterized. To examine the relation between pro-inflammatory responses and TSPO expression, we isolated primary mouse microglia and astrocytes, and confirmed their purity by ICC using cell-type specific markers (Iba1 and GFAP for microglia and astrocytes, respectively) (Fig. 1A, G). And then we treated primary microglia and astrocytes with various TLR ligands, including Pam3CSK4 (TLR1/2), HKLM (TLR2), Poly (I:C) HMW (TLR3), Poly (I:C) LMW (TLR3), LPS (TLR4), ST-FLA (TLR5), FSL-1 (TLR6/2), ssRNA (TLR7) and ODN1826 (TLR9). Most TLR ligands induced the secretion of pro-inflammatory cytokines, TNF-α, IL-6 and CCL2 from microglia (Fig. 1B~D) and astrocytes (Fig. 1H~J). Poly (I:C) LMW, LPS and ST-FLA induced robust pro-inflammatory responses with potent secretion of all three cytokines in both microglia and astrocytes, but others had less consistent effects depending on cell types and cytokines. Next, we checked the effects of the TLR ligands on the expression levels of TSPO in microglia and astrocytes. As shown in Fig. 1E and 1F, Poly (I:C) HMW, Poly (I:C) LMW and LPS significantly increased TSPO expression, while Pam3CSK4, HKLM, ST-FLA, ssRNA and ODN1826 had much lesser effects in microglia. On the other hand, we observed modest up-regulation of TSPO protein levels by the TLR ligands in astrocytes (Fig. 1K and 1L). Therefore, these results suggest that most TLR ligands can elicit pro-inflammatory responses in microglia and astrocytes. But, the effects of the TLRs stimulation on the expression levels of TSPO can be different between microglia and astrocytes as well as among the TLR members.
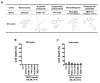
To investigate the pharmacological effects of TSPO ligands on TLR ligands-induced pro-inflammatory responses in microglia and astrocytes, we selected 5 representative TSPO ligands with different structural classes: etifoxine (benzoxazine class), FGIN-1-27 (arylindol acetamide class), PK11195 (isoquinoline carboxamide class), Ro5-4864 (benzodiazepam class) and XBD-17 (phenylpurine acetamide class) (Fig. 2A) [3]. Although TSPO ligands may cause cytotoxic effects in specific types of cells [14], we confirmed that TSPO ligands were not toxic on microglia and astrocytes at the doses used in this study (Fig. 2B, C).
To investigate the effects of TSPO ligands on a variety of TLR ligands-induced pro-inflammatory responses in microglial cells, we pre-treated primary mouse microglia with the TSPO ligands. After 1 h of pre-incubation of TSPO ligands, microglia were stimulated with the TLR ligands and secretions of TNF-α, IL-6 and CCL2 were measured 12 h and 24 h later.
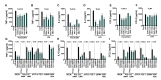
First, we compared the effects of five TSPO ligands against TLR1/2 or TLR4 stimulation. The generations of TNF-α, IL-6 by Pam3CSK4 or LPS were suppressed by etifoxine, FGIN-1-27, PK11195, Ro5-4864 and XBD-173 (Fig. 3A~D). Interestingly, the TSPO ligands suppressed the secretion of CCL2 elicited by Pam3CSK4, but not LPS (Fig. 3E, F). Additionally, we selected two TSPO ligands, etifoxine and PK11195, for further analyses of their effects on other TLR members, since they showed consistent effects against all three cytokines following PamCSK4 or LPS treatment. As shown in Fig. 3G~I, etifoxine and PK11195 attenuated production of TLR ligands-induced pro-inflammatory cytokines. These results indicate that the TSPO ligands inhibit pro-inflammatory responses of microglia triggered by the various TLR ligands.
Next, we tested the effects of TSPO ligands on TLR ligands-induced pro-inflammatory responses in astrocytes following the same experimental scheme as microglia. As shown in Fig. 4A~F, the secretions of TNF-α and IL-6 by Pam3CSK4 or LPS were efficiently attenuated, but not CCL2, by etifoxine, FGIN-1-27, PK11195, Ro5-4864 and XBD-173 in astrocytes. And etifoxine and PK11195 decreased TLR ligands-induced secretion of pro-inflammatory cytokines in astrocytes (Fig. 4G~I). Collectively, these results suggest that TSPO ligands can reduce TLR ligands-stimulated pro-inflammatory responses in astrocytes. However, their overall effects were less substantial than in microglia.
The TSPO ligands have been reported as promising drugs for treatment of the various neurodegenerative diseases involving neuroinflammation [3,7,15]. Due to the increasing interest in the pathophysiological roles of TSPO and therapeutic potential of TSPO ligands, various TSPO ligands belonging to diverse distinct chemical classes have been made [3,7]. Though different classes of TSPO ligands have shown anti-inflammatory effects through inhibition of reactive microglia and astrocytes in various experimental models of neurodegeneration, the comparative studies of the ligands for their efficacy and related signaling pathways in the same cellular model of neuroinflammation was not yet conducted. Furthermore, the distinct pharmacological actions of the TSPO ligands between microglia and astrocytes were not investigated, either. Therefore, we wished to compare the anti-inflammatory activities of the TSPO ligands in microglia and astrocytes. For that purpose, we chose 5 different TSPO ligands representative of the key chemical classes [3]. Glia activation and neuroinflammation were estimated by measuring three well-known pro-inflammatory cytokines: TNF-α, IL-6 and CCL2 [10]. The TNF-α and IL-6 are the amplifiers of inflammatory program, and CCL2 (also called MCP-1) is known to recruit additional immune cells, thereby regulate migration and infiltration of monocytes/macrophages during inflammation [10,16].
To activate microglia and astrocytes, we targeted the TLRs, since accumulating evidence has demonstrated the important roles of the TLRs in the CNS immunity [10]. Previous studies reported that the TSPO ligands suppressed secretion of LPS (TLR4 ligand)-induced pro-inflammatory cytokines [5]. However, it has not been known whether the TSPO ligands are also protective against other TLR ligands-triggered neuroinflammation, and also whether they have similar pharmacological profiles between microglia and astrocytes.
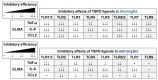
In this report, we found that the TSPO ligands can suppress activation of TLRs-stimulated microglia and astrocytes with different efficacies depending on cell types and TLR members. Interestingly, up-regulation of TSPO protein level was not so robust in astrocytes, compared with the correlative trend of marked increase of TSPO level in microglia activation. These data suggest that the regulatory mechanisms of TSPO expression and their potential roles in neuroinflammation may be different to some degree between microglia and astrocytes. Though TSPO ligands suppressed TLR ligands-induced pro-inflammatory cytokine secretion in both microglia and astrocytes, the TSPO ligands more efficiently inhibited pro-inflammatory responses of microglia than astrocytes, as summarized in Fig. 5. Collectively, our results demonstrated that TSPO ligands are the promising reagents in the suppression of pro-inflammatory responses of both microglia and astrocytes. Further studies will be warranted to understand the mechanisms underlying these cellular differences and to come up with better therapeutic design targeting TSPO for the treatment of neuroinflammation-mediated neurodegenerative conditions.

- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 2006;27:402-409.
- Veiga S, Carrero P, Pernia O, Azcoitia I, Garcia-Segura LM. Translocator protein 18 kDa is involved in the regulation of reactive gliosis. Glia 2007;55:1426-1436.
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 2010;9:971-988.
- Daugherty DJ, Chechneva O, Mayrhofer F, Deng W. The hGFAP-driven conditional TSPO knockout is protective in a mouse model of multiple sclerosis. Sci Rep 2016;6:22556.
- Bae KR, Shim HJ, Balu D, Kim SR, Yu SW. Translocator protein 18 kDa negatively regulates inflammation in microglia. J Neuroimmune Pharmacol 2014;9:424-437.
- Banati RB, Middleton RJ, Chan R, Hatty CR, Kam WW, Quin C, Graeber MB, Parmar A, Zahra D, Callaghan P, Fok S, Howell NR, Gregoire M, Szabo A, Pham T, Davis E, Liu GJ. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat Commun 2014;5:5452.
- Arbo BD, Benetti F, Garcia-Segura LM, Ribeiro MF. Therapeutic actions of translocator protein (18 kDa) ligands in experimental models of psychiatric disorders and neurodegenerative diseases. J Steroid Biochem Mol Biol 2015;154:68-74.
- Kim EJ, Yu SW. Translocator protein 18 kDa (TSPO): old dogma, new mice, new structure, and new questions for neuroprotection. Neural Regen Res 2015;10:878-880.
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 2007;7:353-364.
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res 2006;83:711-730.
- Siegemund S, Sauer K. Balancing pro- and anti-inflammatory TLR4 signaling. Nat Immunol 2012;13:1031-1033.
- Tamashiro TT, Dalgard CL, Byrnes KR. Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. J Vis Exp 2012;:e3814.
- Schildge S, Bohrer C, Beck K, Schachtrup C. Isolation and culture of mouse cortical astrocytes. J Vis Exp 2013;:50079.
- Naoi M, Maruyama W, Yi H. Rasagiline prevents apoptosis induced by PK11195, a ligand of the outer membrane translocator protein (18 kDa), in SH-SY5Y cells through suppression of cytochrome c release from mitochondria. J Neural Transm (Vienna) 2013;120:1539-1551.
- Lee JW, Kim LE, Shim HJ, Kim EK, Hwang WC, Min DS, Yu SW. A translocator protein 18 kDa ligand, Ro5-4864, inhibits ATP-induced NLRP3 inflammasome activation. Biochem Biophys Res Commun 2016;474:587-593.
- Cazareth J, Guyon A, Heurteaux C, Chabry J, Petit-Paitel A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: importance of CCR2/CCL2 signaling. J Neuroinflammation 2014;11:132.



