Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2018; 27(2): 112-119
Published online April 30, 2018
https://doi.org/10.5607/en.2018.27.2.112
© The Korean Society for Brain and Neural Sciences
Aucubin Promotes Differentiation of Neural Precursor Cells into GABAergic Neurons
Miyeoun Song1, Hyomin Kim1, Sujin Park1, Hyockman Kwon3, Insil Joung4 and Yunhee Kim Kwon1,2*
1Department of Life and Nanopharmarceutical Science, Kyung Hee University, Seoul 02447, 2Department of Biology, Kyung Hee University, Seoul 02447, 3Department of Biosciences and Biotechnology, Hankuk University of Foreign Studies, Yongin 17035, 4Department of Biological Sciences, Hanseo University, Seosan 31962, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-961-0844, FAX: 82-2-966-4497
e-mail: kimyh@khu.ac.kr
Abstract
Aucubin is a small compound naturally found in traditional medicinal herbs with primarily anti-inflammatory and protective effects. In the nervous system, aucubin is reported to be neuroprotective by enhancing neuronal survival and inhibiting apoptotic cell death in cultures and disease models. Our previous data, however, suggest that aucubin facilitates neurite elongation in cultured hippocampal neurons and axonal regrowth in regenerating sciatic nerves. Here, we investigated whether aucubin facilitates the differentiation of neural precursor cells (NPCs) into specific types of neurons. In NPCs cultured primarily from the rat embryonic hippocampus, aucubin significantly elevated the number of GAD65/67 immunoreactive cells and the expression of GAD65/67 proteins was upregulated dramatically by more than three-fold at relatively low concentrations of aucubin (0.01 µM to 10 µM). The expression of both NeuN and vGluT1 of NPCs, the markers for neurons and glutamatergic cells, respectively, and the number of vGluT1 immunoreactive cells also increased with higher concentrations of aucubin (1 µM and 10 µM), but the ratio of the increases was largely lower than GAD expression and GAD immunoreactive cells. The GABAergic differentiation of pax6-expressing late NPCs into GABA-producing cells was further supported in cortical NPCs primarily cultured from transgenic mouse brains, which express recombinant GFP under the control of pax6 promoter. The results suggest that aucubin can be developed as a therapeutic candidate for neurodegenerative disorders caused by the loss of inhibitory GABAergic neurons.
Graphical Abstract
Keywords: aucubin, primary neuronal precursor cells, neuronal differentiation, GABAergic neuron, glutamatergic neuron
INTRODUCTION
Human neurodegenerative diseases are caused by the chronic and progressive loss of specific types of neurons. The loss of cholinergic neurons in the cerebral cortex and basal forebrain in Alzheimer's disease (AD), midbrain dopaminergic neurons in Parkinson's disease (PD), and striatal gamma-aminobutyric acidergic (GABAergic) neurons in Huntington's disease initiate these degenerating diseases. Neural precursor cells (NPCs), present in the subgranular zone of the hippocampus and subventricular zone of adult brains, can replace those damaged cells. Therefore, promoting the differentiation of endogenous NPCs into those specific neuron types lost in the degenerating diseases may facilitate neuronal regeneration. Small compounds that have the potential for enhancing the proliferation or differentiation of endogenous NPCs into specific types of neurons can be useful therapeutics for improving neuronal regeneration without surgical methods.
Aucubin (1,4a,5,7a,tetra5hydroxy7(hydroxymethyl) cycopenta (c) pyran-1-y1-b-D-glucopy-ranoside) is an iridoid glycoside, commonly isolated from traditional medicinal herbs [1,2]. It shows primarily anti-inflammatory and protective effects, although various pharmacological effects have been reported [3,4,5]. In the nervous system, aucubin has been shown to have neuroprotective effects by enhancing neuronal survival and reducing apoptotic cells [6,7]. There have been, however, several reports suggesting that aucubin might have effects on facilitating neuronal differentiation in addition to neuronal protection and survival, including our previous studies [8,9,10]. Despite the known effects of aucubin on various biological activities, little is known about its effects on neuronal differentiation. In this study, we investigated whether aucubin enhances differentiation into specific neuronal cell types using NPCs of the hippocampus and cortex, primarily cultured from embryonic rodent brains.
MATERIALS AND METHODS
NPCs were isolated from the rat embryonic day 16 (E16) embryonic brain as described previously [11,12]. Time-pregnant Sprague Dawley rats were purchased from Orient Co., Ltd., a branch of Charles River Laboratories (Gyeonggi-do, Korea). NPCs expressing green fluorescent protein (GFP) were isolated from the transgenic mice expressing GFP under the control of paired box protein 6 (pax6) promoter (pax6-GFP mice), which were kindly offered by Drs. Jiha Kim and James D. Lauderdale, Dept. of Cellular Biology, University of Georgia, Athens, GA 30602, USA [13,14]. Briefly, hippocampal eminence was dissected from the rat E16 embryonic forebrain aseptically using fine forceps under a dissecting microscope. NPCs expressing GFP were isolated from the dorsal forebrain of the E14 embryo of pax6-GFP transgenic mice. The tissues were collected and dissociated mechanically in Ca2+/Mg2+-free Hank's Balanced Salt Solution (HBSS, Cat. No. 88284, Invitrogen) and the cells were plated at 30,000 cells/cm2 on a 60 mm culture dish pre-coated with 15 µg/ml poly-L-ornithine (Cat. No. P4957, Sigma-Aldrich, St. Louis, MO, USA) and 1 µg/ml fibronectin (Cat. No. F0635, Sigma-Aldrich, St. Louis, MO, USA), and allowed to proliferate in serum-free N2 media with basic fibroblast growth factor (bFGF; 10 ng/ml; Cat. No. P15655, R&D system, USA) for 3~4 days up to 70~80% confluences. bFGF is known to stimulate cell division and suppress the differentiation of NPCs [15,16]. To induce differentiation into neurons, NPCs were grown in N2 media with bFGF for an additional 1 day after plating on new dishes and changed with N2 media without bFGF and subsequently incubated for appropriate times (differentiation media). In order to examine the effect of aucubin on neuronal differentiation, NPCs were incubated in differentiation media including various concentrations of aucubin (0, 0.01, 0.1, 1, and 10 µM, Cat. No. 55561, Sigma-Aldrich, St. Louis, MO, USA).
For immunocytochemical assays, NPCs were grown on glass coverslips (Cat. No. 1916-91012, Bellco, USA) for 7 more days for differentiation including aucubin to detect specific neuron markers. NPCs were passaged using 0.05% trypsin–EDTA (Cat. No. 25300054, Gibco by Thermo Fisher Scientific Inc.) on 12 mm glass coverslips (Cat. No. 1916-91012, Bellco, Vineland, NJ, USA). Rat hippocampal NPCs (3×104 cells/cm2) or mouse NPCs from dorsal forebrain (1.5×104 cells/cm2) were grown in N2 media with bFGF for an additional 1 day. Rat hippocampal NPCs were grown for 6 more days and mouse NPCs from dorsal forebrain for 2 more days without fibroblast growth factors (FGF) to detect specific neuronal cell markers (glutamic acid dehydrogenase 65/67 (GAD65/67), vesicular glutamate transporter 1 (vGluT1), and GFP). For immunoblot assays, NPCs (3×104 cells/cm2) were grown on a 60 mm dish for 3 more days in differentiation media with or without aucubin.
NPCs cultured on coverslips were fixed with 4% paraformaldehyde (Sigma) for 30 min and washed with phosphate-buffered saline (PBS). For GABA antibody reactivity, the cells were fixed with 4% paraformaldehyde, 0~0.5% glutaraldehyde, and 0.5% potassium dichromate in 0.1 M phosphate buffer at pH 6.5. After fixing, the cells were incubated with primary antibodies against GFP (rabbit anti-GFP antibody, 1:500, Cat. No. AB3080, Merck KGaA, Darmstadt, Germany), gamma-aminobutyric acid (mouse anti-GABA antibody, 1:1000, Cat. No. ab86186, Abcam), glutamic acid dehydrogenase 65/67 (rabbit anti-GAD65/67 antibody, 1:500, Cat. No. Ab1511, Chemicon International Inc.), and vesicular glutamate transporter 1 (mouse anti-vGluT1 antibody, 1:500, Cat. No. MAB5502, Chemicon International Inc.). Cells were subsequently incubated with FITC- or Alexa fluor-conjugated secondary antibodies (1:200 for each, Molecular Probes). Slides were counterstained with 4,6-di-amidine-2-phenylindole dihydrochloride (DAPI, 1 µg/ml, Cat. No. D9542, Sigma-Aldrich). At least five fields of view (objective magnification: ×200) from an average of five slides per experimental group were selected randomly and neuronal differentiation marker-positive cells were then counted. Stained slides were examined under a confocal laser scanning biological microscope (LSM 510, Carl Zeiss).
NPCs in 60 mm dishes incubated for 3 days in differentiation media with or without aucubin were lysed in 80 µl of ice-cold radioimmunoprecipitation assay (RIPA) buffer (cat # R0278, Sigma-Aldrich) containing a protease inhibitor cocktail and cleared via 10 min of centrifugation at 13,000×g. Following determination of protein concentration using Bradford protein assay kits (Cat. No. B6916, Bio-Rad, Hercules, California, USA), 10~20 µg of proteins were separated on 10 % sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and probed for various neuronal markers and β-actin. The primary antibodies used were mouse anti-vGluT1 antibody (1:400, Chemicon), rabbit anti-GAD65/67 antibody (1:500, Cat. No. PA5-36080, Chemicon), rabbit anti-NeuN antibody (1:5000, Cat. No. ab104225, Abcam) and mouse anti-β-actin antibody (1:1000, Cat. No. sc-47778, Santa Cruz, Delaware, California, USA). Signals were visualized using enhanced chemiluminescence (LumiGLO, KPL Europe, Guildford, UK) as recommended by the manufacturer. The intensity of each band was quantified with a densitometric scanner using an image analysis program.
All quantitative data are expressed as mean±standard deviation. A one-way analysis of variance (ANOVA) using the Bonferroni test was performed to determine significant differences. The assumptions of ANOVA were found to satisfy Levene's test for homogeneity of variance and to pass tests for normality. A value of p<0.05 was considered statistically significant.
RESULTS
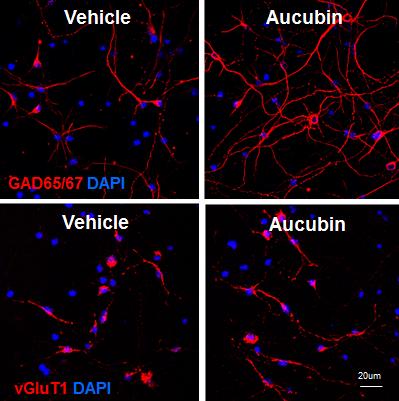
To search for the effect of aucubin on neuronal differentiation, we isolated NPCs primarily from the rat E16 hippocampus when precursors of pyramidal cells initiate proliferation [17,18]. Neuronal differentiation of NPCs was induced by incubating in chemically defined N2 medium without bFGF. In the differentiation condition, NPCs were treated with various concentrations, such as 0.01, 0.1, 1 and 10 µM of aucubin. We investigated whether aucubin facilitates neuronal differentiation into specific types of neuronal cells by examining GAD65/67- and vGluT1-expressing cells using an immunocytochemical assay. GAD65/67, a GABA synthesizing enzyme and vGluT1 are expressed specifically in GABAergic and glutamatergic neurons, respectively, which are the representative inhibitory and excitatory neurons in the mammalian brain. After 7 days of differentiation, GAD immunoreactive cells elongated a number of long neurites with the addition of aucubin, while vGluT1 immunoreactive cells contained mostly one short neurite. The length of the neurites of differentiated cells immunostained with anti-GAD65/67 antibodies seemed to be increased when the aucubin concentration was increased (Fig. 1A and 1C). While the numbers of both GAD65/67 and vGluT1 immunoreactive cells increased statistically significantly in aucubin-treated NPCs compared to NPCs with vehicle-treated control, the increase was much more prominent in GAD65/67 immunoreactive cells (Fig. 1A and 1D). Aucubin treatment at a low concentration of 0.1 µM increased GAD65/67 immunoreactive cells about 1.8 times compared to control, and a bigger increase was observed at 10 µM concentration (Fig. 1A). In contrast, vGluT1 immunoreactive cells increased at most by 1.1-fold with 0.1 µM of aucubin (Fig. 1C and 1D), without a bigger increase at higher concentrations of aucubin. These data indicate that aucubin promotes the neuronal differentiation of NPCs more into GAD65/67-expressing cells than to vGluT1-expressing cells.
To determine whether aucubin promotes neuronal differentiation specific to GABAergic neurons, we examined the protein expression of GAD65/67, vGluT1, and a mature neuron marker, NeuN, in NPCs differentiated with or without aucubin by immunoblotting analysis. Fig. 2 shows that the treatment of aucubin in differentiation conditions upregulated the protein expression of NeuN, GAD65/67, and vGluT1. When the intensity of the expressed proteins was accessed in relation to β-actin, a substantial increase in GAD65/67 expression was detected in 0.01 µM aucubin-treated NPCs and a dose-dependent increase of up to 3.8-fold was observed in the 1 µM concentration of aucubin (Fig. 2A and 2C). However, the increase in vGluT1 expression in NPCs treated with a concentration of aucubin between 0.01 and 10 µM was much less than that of GAD65/67 (Fig. 2C and 2D). NeuN, a mature neuron marker, was also expressed more in differentiated NPCs treated with 1 and 10 µM of aucubin, similar to vGluT1 (Fig. 2B). Taken together, these results suggest that aucubin has a differential effect to promote neuronal differentiation into GABAergic neurons rather than glutamatergic neurons.
Glutamate decarboxylase (GAD65 and GAD67) is responsible for synthesizing GABA, an inhibitory neuronal transmitter. To confirm the effects of aucubin on the cell type differentiation of cortical NPCs, we next examined if aucubin enhances GABAergic differentiation and produces GABA. We isolated cortical NPCs from the dorsal forebrain of pax6-GFP transgenic mice, which express GFP under the control of pax6 promoter. In the differentiation condition, cortical NPCs were treated with the same concentrations of aucubin (0.01, 0.1, 1, and 10 µM) as hippocampal NPCs and the proportion of cells producing GABA was determined by immunocytochemical analysis. As shown in Fig. 3A, treatment with aucubin at various concentrations for 3 days increased the number of pax6-expressing NPCs, determined as GFP-positive cells. Aucubin at a very low concentration of 0.01 µM showed the effect of increasing the differentiation of NPCs, and treatment with 1 µM of aucubin increased the number of GFP-positive NPCs more than two-fold compared to NPCs with vehicle treatment (Fig. 3A and 3B). When the proportion of GABA-producing neurons among DAPI-positive cells was determined by immunostaining using anti-GABA antibodies, increasing values were observed with aucubin treatment during neuronal differentiation (Fig. 3B). In particular, in the differentiation condition including 0.1 µM aucubin, it exhibited a more than 1.5-fold increase over controls in the proportion of GABA-producing neurons. This indicates that aucubin facilitates differentiation into neurons producing GABA, an inhibitory neurotransmitter, during the neuronal differentiation of NPCs.
DISCUSSION
Although not many studies concerning the effects of aucubin on the nervous system have been reported, some studies have shown that aucubin has neuroprotective effects [6,7,19]. Aucubin protects PC12 cells from H2O2-induced apoptosis by modulating the expression of the Bcl-2 family [6,7]. In the rat model of diabetic encephalopathy, showing the duration of the diabetes–related loss of hippocampal neurons in the CA1 and CA2 subfields, the cell densities of surviving neurons in the CA1 region of rats injected intraperitoneally with aucubin were greater than those of non-treated rats [20]. Aucubin has been shown to protect neurons from cell death by changing antioxidative ability and the expression of Bcl-2 and Bax in the rat model of diabetic encephalopathy [7,20]. Thus, aucubin seems to protect neurons by enhancing neuronal survival and reducing apoptotic cells, whereas we recently found that aucubin facilitates the neurite outgrowth of hippocampal NPCs primarily cultured from rat embryos (E16) and axonal elongation in the injured peripheral nervous system [10]. There are also reports that natural lignans and iridoid compounds including aucubin lengthen the neurites of PC12h cells, a cultured cell line of paraneuron [8,9]. This suggests that in addition to neuronal survival and neuro-protection, aucubin may promote the neuronal differentiation of NPCs.
Here, we examined the effects of aucubin on neuronal differentiation into specific cell types using NPC cultures primarily obtained from the rat embryonic hippocampus. We observed that the treatment of aucubin at a concentration of 0.01 to 10 µM increased cells expressing GAD65/67, a neuronal marker of GABAergic cells, about two-fold with an immunostaining assay. Furthermore, immunoblot analysis showed that the expression of GAD65/67 in differentiating neurons was elevated about 3.8-fold with 1 µM of aucubin. Interestingly, the upregulation of GAD65/67 expression was more prominent compared to that of vGluT1 expression, which was about 1.8-fold at most, indicating that aucubin promotes the neuronal differentiation of NPCs more preferentially to GABAergic cells than to glutamatergic cells. In contrast, the increase in vGluT1 expression was similar to that of NeuN, suggesting that the elevation of vGluT1 expression is due to neuronal differentiation increased by aucubin treatment.
This notion was further supported by the experiment in cortical NPCs expressing pax6, showing that the portion of GABA, an inhibitory neurotransmitter producing cells among differentiating neurons of NPCs, was increased more than 1.8-fold with 0.1 µM of aucubin. The cortical NPCs expressing pax6 were isolated from pax6-GFP transgenic mice that express recombinant GFP under the control of pax6 promoter. pax6 is a transcription factor function in late-stage NPCs. Thus, we confirmed that aucubin facilitates the differentiation of pax6-expressing NPCs to GABA-producing cells in addition to the survival of NPCs.
During mammalian neuronal development, GABAergic interneurons are generated within the ganglionic eminences and populate all regions of the forebrain, including the olfactory bulb, cortex, and hippocampus. The molecular mechanisms that promote survival, migration, and subtype specification of GABAergic neurons are under investigation, but it is likely that Mash1 and Dlx transcription factors to direct GABAergic neuron differentiation [21,22]. During GABAergic differentiation a sequential cascade of Dlx expression is induced and maintained; Dlx2 followed by Dlx1, Dlx5 and Dlx6 [23]. Dlx1 and 2−/− mutants have reduced GAD expression, a block in tangential migration, abnormal neurite morphogenesis, decreased neuronal survival and synaptogenesis and reduced glutamatergic input to hippocampal interneurons [21]. The expression of the transcription factors inducing GABAergic differentiation may be stimulated by extrinsic factors, such as BDNF and HRG, as known to increase GABAergic cells. How aucubin promotes GABAergic differentiation is not clear yet, but it may increase the production of extrinsic factors, or stimulate the signal transduction pathways to regulate the expression of Mash 1 and Dlxs.
Recently, aucubin has been reported to increase neuronal survival in CA1 and CA3 hippocampal regions and ameliorate damage by inducing autophagy and inhibiting necroptosis in the hippocampus of lithium-pilocarpine-induced status epilepticus rat model [24]. Our results suggest that aucubin may ameliorate damage by facilitating the differentiation of NPCs near the hippocampal alveus into GABAergic neurons, because GABAergic neurons specifically decrease in the hippocampus of lithium-pilocarpine-induced status epilepticus rat models.
Many neurodegenerative diseases are caused by the loss of specific types of neurons, such as glutamatergic, cholinergic, dopaminergic and GABAergic neurons [25]. AD is thought to be caused partly by hippocampal hyperactivity as a result of the loss of inhibitory interneuron function [26]. In rodent models of AD, significant decreases in the number of GABAergic interneurons are one of the conspicuous alterations seen in the aged hippocampus. In an attempt to treat neurodegenerative disorders including AD, Parkinson's disease, and chronic epilepsy, GABAergic cell grafting has drawn much attention and has been tested in animal models [27,28]. However, it may be useful to find small compounds with the purpose of using them as therapeutic candidates to treat neurodegenerative disorders when the compounds generate the neuronal types necessary for the damaged brain from the patient's own NPCs, instead of grafting exogenous NPCs with surgical methods.
With our present results we demonstrate that aucubin strongly promotes GABAergic differentiation from NPCs. Additional studies are needed to investigate the effects of aucubin using NPCs that originate from humans. Despite these limitations, our data imply that aucubin may be a useful therapeutic compound for treating neurodegenerative disorders caused by the loss or impairment of inhibitory GABAergic neurons.
Figures



References
- Bernini R, Iavarone C, Trogolo C. 1-O-β-D-glucopyranosyleucommiol, an iridoid glucoside from Aucuba japonica. Phytochemistry 1984;23:1431-1433.
- Suomi J, Sirén H, Wiedmer SK, Riekkola ML. Isolation of aucubin and catalpol from Melitaea cinxia larvae and quantification by micellar electrokinetic capillary chromatography. Anal Chim Acta 2001;429:91-99.
- Kim MB, Kim C, Chung WS, Cho JH, Nam D, Kim SH, Ahn KS. The hydrolysed products of iridoid glycosides can enhance imatinib mesylate-induced apoptosis in human myeloid leukaemia cells. Phytother Res 2015;29:434-443.
- Park KS. Aucubin, a naturally occurring iridoid glycoside inhibits TNF-α-induced inflammatory responses through suppression of NF-κB activation in 3T3-L1 adipocytes. Cytokine 2013;62:407-412.
- Young IC, Chuang ST, Hsu CH, Sun YJ, Liu HC, Chen YS, Lin FH. Protective effects of aucubin on osteoarthritic chondrocyte model induced by hydrogen peroxide and mechanical stimulus. BMC Complement Altern Med 2017;17:91.
- Xue HY, Niu DY, Gao GZ, Lin QY, Jin LJ, Xu YP. Aucubin modulates Bcl-2 family proteins expression and inhibits caspases cascade in H2O2-induced PC12 cells. Mol Biol Rep 2011;38:3561-3567.
- Xue HY, Gao GZ, Lin QY, Jin LJ, Xu YP. Protective effects of aucubin on H2O2-induced apoptosis in PC12 cells. Phytother Res 2012;26:369-374.
- Yamazaki M, Hirota K, Chiba K, Mohri T. Promotion of neuronal differentiation of PC12h cells by natural lignans and iridoids. Biol Pharm Bull 1994;17:1604-1608.
- Yamazaki M, Chiba K, Mohri T. Neuritogenic effect of natural iridoid compounds on PC12h cells and its possible relation to signaling protein kinases. Biol Pharm Bull 1996;19:791-795.
- Kim YM, Sim UC, Shin Y, Kim Kwon Y. Aucubin promotes neurite outgrowth in neural stem cells and axonal regeneration in sciatic nerves. Exp Neurobiol 2014;23:238-245.
- Lim JS, Cho H, Hong HS, Kwon H, Mook-Jung I, Kwon YK. Upregulation of amyloid precursor protein by platelet-derived growth factor in hippocampal precursor cells. Neuroreport 2007;18:1225-1229.
- Jo AY, Park CH, Aizawa S, Lee SH. Contrasting and brain region-specific roles of neurogenin2 and mash1 in GABAergic neuron differentiation in vitro. Exp Cell Res 2007;313:4066-4081.
- Kim J, Lauderdale JD. Analysis of pax6 expression using a BAC transgene reveals the presence of a paired-less isoform of pax6 in the eye and olfactory bulb. Dev Biol 2006;292:486-505.
- Kim J, Lauderdale JD. Overexpression of pairedless pax6 in the retina disrupts corneal development and affects lens cell survival. Dev Biol 2008;313:434-454.
- Kitchens DL, Snyder EY, Gottlieb DI. FGF and EGF are mitogens for immortalized neural progenitors. J Neurobiol 1994;25:797-807.
- Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J Neurosci 2003;23:240-251.
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature 1965;207:953-956.
- Park CH, Kang JS, Kim JS, Chung S, Koh JY, Yoon EH, Jo AY, Chang MY, Koh HC, Hwang S, Suh-Kim H, Lee YS, Kim KS, Lee SH. Differential actions of the proneural genes encoding Mash1 and neurogenins in Nurr1-induced dopamine neuron differentiation. J Cell Sci 2006;119:2310-2320.
- Xue HY, Lu YN, Fang XM, Xu YP, Gao GZ, Jin LJ. Neuroprotective properties of aucubin in diabetic rats and diabetic encephalopathy rats. Mol Biol Rep 2012;39:9311-9318.
- Xue HY, Jin L, Jin LJ, Li XY, Zhang P, Ma YS, Lu YN, Xia YQ, Xu XP. Aucubin prevents loss of hippocampal neurons and regulates antioxidative activity in diabetic encephalopathy rats. Phytother Res 2009;23:980-986.
- Pla R, Stanco A, Howard MA, Rubin AN, Vogt D, Mortimer N, Cobos I, Potter GB, Lindtner S, Price JD, Nord AS, Visel A, Schreiner CE, Baraban SC, Rowitch DH, Rubenstein JLR. Dlx1 and Dlx2 promote interneuron gaba synthesis, synaptogenesis, and dendritogenesis. Cereb Cortex 2017;:1-19.
- Yun K, Fischman S, Johnson J, Hrabe de, Weinmaster G, Rubenstein JL. Modulation of the notch signaling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development 2002;129:5029-5040.
- Liu JK, Ghattas I, Liu S, Chen S, Rubenstein JL. Dlx genes encode DNA-binding proteins that are expressed in an overlapping and sequential pattern during basal ganglia differentiation. Dev Dyn 1997;210:498-512.
- Wang J, Li Y, Huang WH, Zeng XC, Li XH, Li J, Zhou J, Xiao J, Xiao B, Ouyang DS, Hu K. The protective effect of aucubin from eucommia ulmoides against status epilepticus by inducing autophagy and inhibiting necroptosis. Am J Chin Med 2017;45:557-573.
- Stoll EA. Advances toward regenerative medicine in the central nervous system: challenges in making stem cell therapy a viable clinical strategy. Mol Cell Ther 2014;2:12.
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell 2012;148:1204-1222.
- Shetty AK, Turner DA. Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy. J Neurosci 2000;20:8788-8801.
- Tyson JA, Anderson SA. GABAergic interneuron transplants to study development and treat disease. Trends Neurosci 2014;37:169-177.



