Articles
Article Tools
Stats or Metrics
Article
Review Article
Exp Neurobiol 2018; 27(6): 453-471
Published online December 28, 2018
https://doi.org/10.5607/en.2018.27.6.453
© The Korean Society for Brain and Neural Sciences
Implantable Neural Probes for Brain-Machine Interfaces ? Current Developments and Future Prospects
Jong-ryul Choi1, Seong-Min Kim2,3, Rae-Hyung Ryu4, Sung-Phil Kim5, and Jeong-woo Sohn2,3*
1Medical Device Development Center, Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF), Daegu 41061, Korea.
2Department of Medical Science, College of Medicine, Catholic Kwandong University, Gangneung 25601, Korea.
3Biomedical Research Institute, Catholic Kwandong University International St. Mary's Hospital, Incheon 21711, Korea.
4Laboratory Animal Center, Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF), Daegu 41061, Korea.
5Department of Human Factors Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Korea.
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-32-280-6523, FAX: 82-32-280-6510
e-mail: jsohn@ish.ac.kr
Abstract
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
A Brain-Machine interface (BMI) allows for direct communication between the brain and machines. Neural probes for recording neural signals are among the essential components of a BMI system. In this report, we review research regarding implantable neural probes and their applications to BMIs. We first discuss conventional neural probes such as the tetrode, Utah array, Michigan probe, and electroencephalography (ECoG), following which we cover advancements in next-generation neural probes. These next-generation probes are associated with improvements in electrical properties, mechanical durability, biocompatibility, and offer a high degree of freedom in practical settings. Specifically, we focus on three key topics: (1) novel implantable neural probes that decrease the level of invasiveness without sacrificing performance, (2) multi-modal neural probes that measure both electrical and optical signals, (3) and neural probes developed using advanced materials. Because safety and precision are critical for practical applications of BMI systems, future studies should aim to enhance these properties when developing next-generation neural probes.
Graphical Abstract
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
Keywords: Implantable neural probes, Brain-machine interface, Multi-channel electrodes, Neural probes with advanced materials
INTRODUCTION
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
Brain–machine interfaces (BMIs) enable direct communication between the brain and machines [1,2]. Due to advancements in information and communication technology, BMIs have gained attention for their promising applications in medical, industrial, and household settings [3,4,5,6].
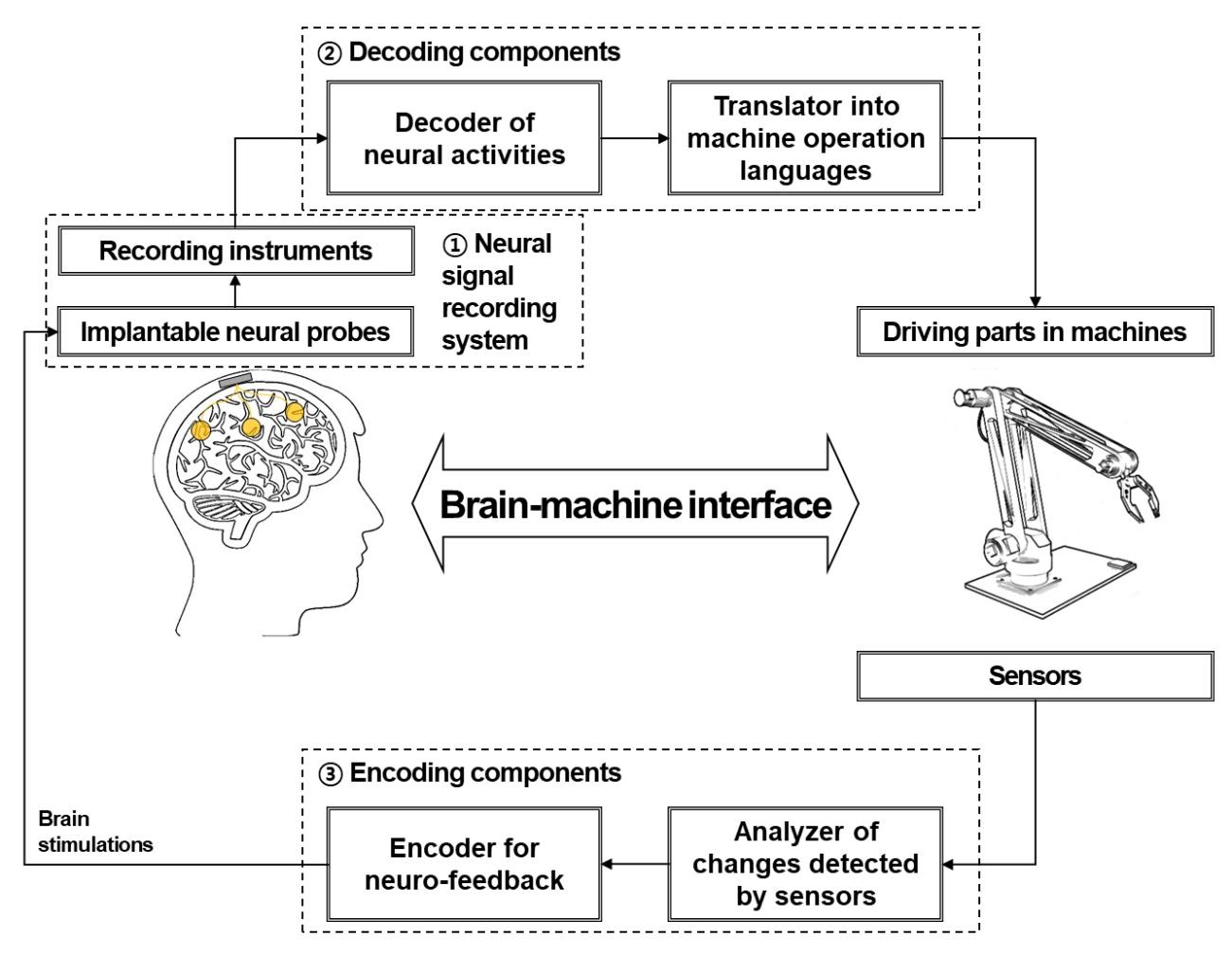
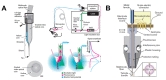
In a unidirectional BMI, the system consists of three components: devices used to record neural signals, components used to analyze the signals, and devices used to provide commands to operate the machine, as illustrated in Fig. 1. In a bidirectional BMI, additional components are required to provide feedback from the machine to the brain [7,8]. While both invasive and non-invasive methods have been developed for the acquisition of neural signals, the present review focuses on implantable neural probes. Thus, hereafter, we will use the terms “neural probes” and “invasive methods” interchangeably.
Electroencephalography (EEG) is widely utilized in non-invasive BMI systems due to its high temporal resolution, which makes it useful for mapping associations between EEG signals and cognitive function [9,10,11]. For example, NeuroSky, Inc. (San Jose, CA, USA) introduced an EEG-based brain–medicine interfacing headset for use in healthcare settings. In addition, Emotiv, Inc. (San Francisco, CA, United Sates) provides a 14-channel system for measuring EEG and other bio-signals for use in brain–computer interfacing games and neurofeedback treatment. Functional near-infrared spectroscopy (fNIRS) and magnetoencephalography (MEG) have also been used to develop non-invasive BMI systems [12,13,14,15]. However, non-invasive neural methods are limited in that neural signals from non-invasive probes are typically insufficient for complicated tasks that require a high degree of freedom, such as robot control [16,17,18,19]. For this reason, implantable neural probes are preferred for BMI systems that demand accurate controls and adjustments (e.g., neuroprosthetic devices).
Implantable neural probes are defined as devices implanted into the brain or other nervous tissues. Communication between neurons in the brain occurs via electrical and chemical signals. In most cases, electrical signals are the main source of information in BMI systems. In particular, single-unit activity (i.e., spikes) is regarded as most appropriate for extracting meaningful information, such as movement-related activity [20]. While non-invasive methods record neural activity through different media such as the dura matter, cerebral spinal fluid (CSF), and skull, implanted neural probes can record extracellular activity or local field potentials closer to neurons. To maximize the signal-to-noise ratio, the conductive material is often exposed at the end of the electrode, the shank of which is insulated with non-conductive material. Typically, single-wire electrodes [21] and glass micropipette electrodes are used in electrophysiological studies [22,23]. Recent advancements have enabled the development of implantable neural probes with the technical characteristics necessary for practical BMI applications, which include high spatiotemporal resolution and high signal-to-noise ratio. Biocompatibility, biochemical stability, and miniaturization are also important, as neural probes must be inserted into the brain. Indeed, implantable multi-array neural probes with these characteristics have been developed and applied for research, diagnosis, and treatment purposes. Representative neural probes in this category include the tetrode, Utah array, and Michigan probe.
In the present report, we review the features of these three implantable neural probes and their applications in BMI systems. Furthermore, we describe several novel technical approaches for improving the measurement of neural signals in BMI systems: (1) decreasing the invasiveness of implantable neural probes while maintaining performance, (2) using multi-modal probes to measure both electrical and optical signals from neurons, and (3) using flexible neural probes to enhance signal quality and biocompatibility. We further discuss (4) novel fabrication techniques and materials that can be utilized to improve neural probes. By exploring recent developments in implantable neural probe technology, we offer insight into the performance required to create practical, efficient, and accurate BMI systems.
APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
BMIs have been successfully applied in the field of neuroprosthetics, enabling patients with paralysis to control robot arms using their thoughts. The underlying neural principle of this phenomenon involves population vectors, in which each neuron “votes” for the intended movement [20]. Increasing the number of neuronal signals increases the likelihood of movement in the population vector. Thus, probes including a high number of channels are suitable for BMIs. In this section, we discuss three popular implantable neural probes with high numbers of channels.
TETRODE
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
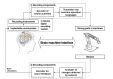
The tetrode is widely utilized to record extracellular electrical potentials in neural systems. As the prefix (tetr-) indicates, the tetrode consists of four electrodes at which neuronal signals are detected from slightly different spatial points originating from the same source. Such electrodes are typically constructed from a platinum–tungsten alloy and insulated with quartz coating. The metal end of the electrode is exposed to acquire electrical signals. The diameter of each electrode at the metal end is usually less than 30 µm, and may be up to100 µm at the coated end. The coating functions to minimize the interference of electrophysiological signals across electrodes. As illustrated in Fig. 2A, a single tetrode can measure the extracellular potentials of approximately 1,100 neurons within a 140-µm radius in the rat cortex [24,25,26]. One main advantage of this method over single-channel electrodes is that users can classify the extracellular potentials of adjacent neurons using a clustering approach [27,28,29]. The extracellular potentials of each neuron are detected by the four electrodes in the tetrode with different temporal points and waveforms because due to the difference in spatial distance between each electrode and the neuron. Spikes from multiple neurons can thus be separated in the post-processing stage. However, the tetrode cannot provide direct measurements of spatially multi-dimensional extracellular potential distributions unless multiple tetrodes are precisely implanted and arranged at regular intervals. Recently, several research groups have developed multi-tetrode arrays to compensate for these shortcomings [30,31].
The tetrode has been used as an implantable neural probe in BMI platforms, especially in small animals. Giszter et al. described a neurorobotic platform, which consisted of a tetrode-based neural recording module and a three-dimensional (3D) robotic module, for use with spinal or cortical prosthetics in small animals (i.e., frogs and rats) [32]. Song et al. developed a novel BMI system to identify movement-related information in the rat cortex during treadmill walking [33]. Using decoded neural activity acquired from six implanted tetrodes, specific regions related to proximal limb and trunk movements were identified in the rat motor cortex. In a follow-up study, Song and Giszter introduced a pelvis-attached robot that can be controlled by cortical neural signals using a multi-tetrode array implanted in the rat brain (Fig. 2B) [34]. Bender et al. investigated that activity of central complex neurons in the insect brain during walking using a tetrode-based system [35]. The authors reported a close association between movements and sensory responses, providing insight into the feasibility of such methods for use in larger animals with more developed brains. Additional groups have also reported advancements in post-processing techniques that enable sorting of spikes in practical BMI systems. For example, Oweiss introduced a novel spatiotemporal signal processing method to improve data compression and reduce latency in multi-tetrode BMI systems [36]. Kubo et al. further investigated the 3D distributions of neurons based on multi-site neural activity using the tetrode [37].
UTAH ARRAY
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
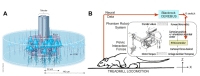
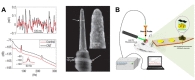
Advancements in semiconductor fabrication processes during the late 20th century have led to the development of multi-channel arrays that perform better than single-channel electrodes in multiple respects. The Utah array is a commercially available intracortical electrode array consisting of up to 100 silicon needle-shaped electrodes, which are produced via microscale fabrication techniques such as thermomigration, a combination of mechanical and chemical micromachining, metal deposition, and encapsulation with a polymer made of imide monomers [38]. Due to the large number of electrodes, the Utah array has been mostly used in large animals, especially non-human primates (Fig. 3A). Velliste et al. investigated the ability of a BMI system to provide neuroprosthetic arm control via cortical motor activity in rhesus monkeys (
The Utah array and its recording systems have been approved for clinical applications by the United States Food and Drug Administration (FDA). Several clinical trials of Utah array-based BMI systems involving human patients have been conducted. Simeral et al. reported that one patient with tetraplegia could control a computer cursor (including point-and-click functions) based on neural signals from the motor cortex [45]. Pandarinath et al. analyzed neural population dynamics during movement in two patients with amyotrophic lateral sclerosis (ALS) as they attempted to use their finger to move a computer cursor [46]. One year after implantation, the system still produced adequate signals for neural cursor control (Fig. 3D) [47] or virtual typing [48]. A more challenging task was performed by a human subject with Utah array implantation. Wodlinger et al. developed a BMI system for the control of an anthropomorphic robot arm and hand with 10 degrees of freedom [49]. Some studies have also indicated that BMI systems can bypass the spinal cord circuit to recover hand function in select patients. Bouton et al. introduced a platform for controlling a neuromuscular electrical stimulation sleeve using neural signals from an implanted Utah array, enabling the patient to perform accurate and continuous movements (e.g., grasp-pour-and-stir tasks) [50]. Ajiboye et al. developed an interfacing platform between intracortical neural signals recorded by two Utah arrays and functional electrical stimulation of peripheral muscles to restore arm and hand movements in patients with paralysis [51]. As experiments of tactile feedbacks has been successful in non-human primate, this type of research has begun to be applied to human subject. As tactile feedback experiments using Utah arrays have been successful in non-human primates, researchers have begun to apply such systems in human patients. For example, Flesher et al. stimulated the somatosensory cortex using Utah arrays to recover tactile sensation in human patients [52].
MICHIGAN PROBE
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
The previous two neural probes enable users to acquire neural activity from different cortical areas. However, they are limited in their ability to target deep neural structures in the axial direction [53,54]. In the late 1970s, the first prototype of Michigan probe, a multi-channel depth electrode prototype, was developed based on electron-beam lithographic techniques [55], which was before production of Utah array. Several preclinical studies involving small animals have demonstrated the safety of long-term implantation for the Michigan probe, suggesting that such probes can be utilized in BMI systems [56,57,58]. Electrodes in the Michigan probe (2 to 15 mm) are longer than those in the Utah array (0.5 to 1.5 mm for research). Thus, the Michigan probe may be more suitable when recording from deeper cortical structures.
Vetter et al. applied the Michigan probe to examine extracellular neural activity in the motor cortex of rats using a BMI system [56]. In a preclinical study on the control of emotions and memories, the Michigan probe was used to measure neural activity and provide electrical stimulation. Frost et al. investigated neural activity in the hindlimbs of rats with spinal cord injuries using a 16-channel Michigan probe and explored treatment methods based on neurostimulation [59]. Guggenmos et al. investigated the use of a neural interfacing system to recover neural function after brain injuries in small animals (i.e., rats) demonstrating that the missing brain functions can be restored using a brain-machine-brain interface as described in Fig. 4 [60]. Such findings suggest that neuroprosthetics can successfully applied in the treatment of various neural diseases. Michigan probes have also been validated in non-human primates [61], supporting the notion that such probes can be used to develop neuroprosthetic systems for use in humans.
RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
In the previous session, we described the most widely used implantable neural probes in BMI applications. While the electrical characteristics, biological compatibility, and stability of these probes are sufficient, researchers have attempted to improve their impedance, flexibility, wireless communication, recording area, and accuracy to ultimately improve BMI systems. In this section, we present an overview of recent studies that have responded to these technical challenges for practical BMI applications.
NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
Electrocorticography (ECoG) is used to monitor signals from the cerebral cortex using electrodes placed on the surface of the brain (subdural) or dura matter (epidural). In contrast to implanted neural probes, which rely on single-unit activity, ECoG relies on local field potentials (LFPs). Although a craniotomy is still required to implement ECoG, no brain scarring occurs because the ECoG electrodes are not inserted into the brain tissue. ECoG provides more accurate neural signals than non-invasive approaches because due to direct (subdural) or close (epidural) contact with brain tissue. Since ECoG has clear advantages in terms of neural signal quality when compared with current non-invasive methods, it is widely used in the development of minimally invasive BMI systems [62,63,64,65]. Neural responses can be recorded from multiple sites in the human brain with high spatiotemporal resolution following tactile stimulation, supporting the use of ECoG in medical BMI applications [66]. For example, Wang et al. recorded activity in the human motor cortex during individual finger movement using micro-ECoG electrodes [67]. Subsequent studies demonstrated that a 32-channel ECoG electrode grid could be used to record LFPs in the sensorimotor cortex of a participant with tetraplegia [68]. Shin et al. introduced method for decoding muscle activation from neural activity based on ECoG signals in the motor cortex of non-human primates [69]. In this study, they investigated the potential for several advanced ECoG electrodes for use as neural probes in BMI systems.
Microfabrication techniques have been employed to develop high-resolution ECoG electrodes. Rubehn et al. developed a flexible, high-resolution (252 channels) ECoG electrode array to measure neural activity in the human brain by optimizing designs and implementing high-resolution MEMS fabrication techniques [70]. Henle et al. developed a microscale ECoG electrode array using a laser-based high-speed and high-resolution fabrication method [71]. Due to their biocompatibility, these types of electrode arrays are promising for use in various long-term,
Advancements in ECoG recording systems have occurred with regard to both detection and transmission. Indeed, several studies have suggested that wireless ECoG recording systems can be implemented in BMI systems. For instance, Charvet et al. developed a promising wireless 64-channel neural probe for the acquisition of neural activity as recorded via ECoG [76]. Similarly, wireless microscale ECoG neural probes have been used to investigate reach-and-grasp movements in non-human primates [77]. In addition, Chang and Chiou developed a chronic ECoG system that can be used to measure neural activity without communicating wires or batteries [78]. Such findings suggest that this type of neural probe can be integrated not only in freely moving animals, but also in humans.
Based on our review of studies involving high-performance ECoG with a large number of channels, we conclude that ECoG would be advantageous in BMI applications due to its relatively low level of invasiveness [79] and the potential for long-term LFP recording [80]. In addition, ECoG electrodes can be applied to brain regions of various shapes, enabling the application of BMI systems for various functions [74,75]. We expect that advances in ECoG electrodes will further facilitate BMI applications.
Some research groups have introduced flexible, syringe-injectable electronic devices for the measurement of chronic
A stent is a medical tube that can be implanted into the vessels to maintain an opening for blood flow. Stents are widely utilized in the treatment of arteriosclerosis since its appearance [87], as they are significantly less invasive than traditional methods [88,89]. To achieve minimal invasiveness and long-term biocompatibility, Oxley et al. developed an endovascular stent-electrode array, as pictured in Fig. 5C [90]. Stent-electrode arrays can be installed by inserting a catheter into the appropriate cerebral blood vessel. Accurate induction of each electrode array position can be acquired using x-ray angiographic fluorography. One preclinical study reported that stent-electrode arrays with multiple neural probes could be used for the long-term measurement of somatosensory evoked potentials in the sheep brain. The feasibility and sustainable biocompatibility of long-term/chronic neural interfaces in brain blood vessels have been confirmed via x-ray micro-tomography and histological assays [91]. Recent studies have demonstrated that endovascular stent-electrode arrays can be used to acquire vascular ECoG signals [92] and detect electrochemical changes via impedance spectroscopy [93]. Despite such advancements, no studies have demonstrated that state-of-the-art stent electrodes can provide immediately usable neural signals (e.g., movement-related activity) for BMI applications. Nonetheless, researchers have successfully acquired somatosensory evoked potentials from stent electrodes implanted in the superficial cortical vein overlying the motor cortex in sheep via catheter angiography [90]. Therefore, future studies may be able to harness movement-related information conveyed to stent electrodes to enhance BMI development.
The use of physical wires inside the brain to acquire neural signals is associated with several disadvantages, such as the risk of infection and reduced freedom of movement. To overcome these shortcomings, researchers have investigated the application of wireless neural recording techniques to BMI systems. For instance, Schwarz et al. developed a wireless multi-channel platform for monitoring neural activity, which was applied successfully in freely moving non-human primates [94]. This system, which resembles a crown, consists of hundreds to thousands of microwire electrodes, a wireless recording/transmitting module, and a battery. In addition, the same research group introduced a wheelchair robot that could be controlled based on neural activity recorded wirelessly from an onboard monkey [95]. Similarly, Libedinsky et al. investigated the application of a robotic vehicle independently moved by neural signals using a 100-channel wireless probe. Su et al. introduced a wireless implantable recording/stimulating probe for bidirectional BMIs, as described in Fig. 5D [96]. This latter probe is advantageous due to its small size and ability for remote charging.
Researchers have also investigated the application of ultrasound technology as a carrier of neural signals from medium-depth tissue. Such “neural dust” systems are advantageous in that they are able to send and receive information non-invasively in certain areas of the brain. Seo et al. investigated a neural dust system consisting of recording electrodes, a piezoelectric ultrasound generator, drive electrodes, and a wireless cortical recording platform [97]. In this system, an external ultrasound probe sends out an echo with a specific waveform, following which a reflected wave (i.e., neural signal) is emitted from the system. This neural signal can be isolated by examining the specific waveform transmitted and the backscattered signal. Preliminary studies designed to measure neural activity in the peripheral nervous system using a neural dust system in rats have demonstrated the potential of these systems for application in BMI technologies.
We anticipate that advancements in the following areas will promote the use of wireless neural electrodes in practical BMI applications: ultra-low power consumption, high signal-to-noise ratio, sufficient maximum communication distance for receiving and transmitting data, and channel number [76,98]. Furthermore, wireless BMI systems must be designed to prevent malfunctions by erasing noise from the external environment, to have an efficient means of changing the battery, and to function for a long period of time on single charge [99,100].
MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
While direct electrical measurements can be obtained using implantable neural probes as described in previous sections, optical measurement methods are also utilized to record neural activity at the level of single neurons and neuronal assemblies. Optical methods rely on indices such as the influx of calcium ions (Ca2+) and changes in voltage. Since changes in Ca2+ can represent neural activity, several optical indicators have been applied in studies ranging from
Electrical and optical signals can be measured without interference from one another because their frequency domains are different. For this reason, several prototypes of multi-modal neural probes have been developed to measure both electrical and optical activity in preclinical studies [121, 122]. LeChasseur et al. developed a micro-probe, which consists of a dual-core optical fiber and a 50-µm electrical wire probe for recording both electrical and optical neural activities, as illustrated in Fig. 6A [123]. In a comparative study, the authors recorded optical and electrical neural signals from the same neuron in rats, observing that the two modalities were complementary and highly correlated with one another. Anikeeva et al. developed an optetrode, which is composed of a 200-µm single-channel optical fiber and a tetrode, to investigate optical stimulation and neural responses (recorded by the tetrode), as described in Fig. 6B [124]. The optetrode detected multi-unit neural activity and transient changes when optical stimulation was provided at different frequencies. Voigts et al. investigated an ultralight weight neural probe (i.e., FlexDrive) consisting of a single-core optical fiber and 16, 32, or 64-channel electrodes for studies of multi-dimensional neural responses to optogenetic stimulation [125]. Kwon and colleagues combined a transparent ECoG electrode array and an optical illumination device for optogenetic stimulation [126]. In another preliminary
Based on previous research, we expect the following benefits to be associated with the use of multi-modal neural probes that combine optical and electrophysiological modalities in BMI systems. When using neural probes to record both optical and electrical neural signals, cross-validation of fidelity between the two signals can increase signal reliability. In particular, since the noise sources of the two signals are likely to be different, one signal can be used when another signal is perturbed. In the context of BMI applications, multi-modal probes may be useful when the patient is in an electromagnetically noisy environment. Such probes may also be advantageous for advanced BMI systems that require precise stimulation and minimal interference between signals. However, few optogenetic genes have been identified and cleared for use in humans due to safety concerns [137,138]. Genetic engineering remains quite challenging due to its unknown long-term impact on humans, although it is expected that these technologies will be utilized in various BMI systems once issues regarding safety and stability have been addressed.
NEURAL PROBES WITH ADVANCED MATERIALS
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
In order for successful implantation in the human brain, neural probes must exhibit sufficient biocompatibility, safety, stability, and electrical performance. However, electrodes composed of classic materials such as iridium oxide and platinum are limited in their ability to meet these conditions. To overcome these limitations, advanced materials with special functions and properties including metals, inorganic materials, and polymers have been applied to the development of neural probes.
Carbon nanotubes (CNTs) are cylindrically structured allotropes of carbon that are stronger and have better elasticity and electrical properties than more traditional materials [139,140]. As such, various studies have aimed to develop neural probes using CNTs. Wang et al. designed a microelectrode array composed of CNTs, reporting significant improvements in the charge injection limit when CNT electrodes were used to stimulate cultured hippocampal neurons [141]. Keefer et al. developed a CNT coating to enhance charge transfer in microelectrode arrays [142]. In this study, the authors obtained stronger neural activity from CNT-coated electrodes in the primate visual cortex, with an improvement factor of 7.4 dB (Fig. 7A). Guitchounts et al. developed 16-channel carbon fiber neural probes with which they could acquire long-term chronic neural signals due to the strength and stability of the carbon fiber [143]. In addition, previous studies have demonstrated the successful integration of a soft CNT fiber microelectrode array in long-term bidirectional neural interfaces. Such findings suggest that this system can be used to develop high-performance and biocompatible BMI devices and neuroprosthetic instruments [144]. Graphene, a two-dimensional carbon allotrope, can also be utilized to improve the performance of neural probes due to its mechanical stiffness, flexibility, and superior electrical properties [145]. For instance, Chen et al. designed flexible graphene microprobes that can be applied to record electrical signals associated with neural or cardiac activity [146]. Graphene-based flexible electrodes have also been fabricated to acquire ECoG signals by improving contact with the brain. Additional studies have indicated that graphene can be used to investigate changes in neural activity due to optogenetic stimulation due to its high transparency [147]. Similarly, Kuzum et al. developed a graphene electrode array with high flexibility and transparency: They acquired electrical neural signals and fluorescence images indicating changes in calcium flux using the same electrode [148]. Furthermore, graphene-based electrodes can be integrated into multi-functional neural probes. For instance, Liu et al. introduced neural probes that consist of graphene-oxide and gold-oxide electrodes, as illustrated in Fig. 7B [149]. This novel probe can be used to obtain measurements of both electrophysiological neural signals and electrochemical information via cyclic voltammetry. Indeed, this type of electrode can be used to monitor neural activity and various electrochemical changes after the induction of brain damage due to photo-thrombosis. Apollo et al. also investigated the application of needle-type flexible neural probes made from graphene oxide for use in bidirectional neural interfaces [150].
Due to the development of efficient techniques for fabricating and manufacturing nanoscale structures and particles, nanotechnology has quickly become relevant to biological and biomedical applications Indeed, nanoscale structures and particles have been applied to improve neural probes. Park et al. developed a nanoporous platinum electrode that can be used to record neural signals [151]. In this study, the authors reported that the nanoporous electrodes were associated with improvements in electrical properties, impedance, and charge injection limits when compared with commercially available alternatives such as platinized platinum and iridium oxide electrodes. Other studies have demonstrated the enhanced biocompatibility of gallium phosphide nanowire electrodes [152]. These nanowire electrodes were tested in the rat primary somatosensory cortex. Abidian and colleagues introduced nanostructured polymer-coated conducting electrodes with enhanced electrical properties, improved mechanical adhesion, and better neuronal attachment capabilities [153]. Piret et al. developed boron-doped diamond electrodes with three dimensionally fabricated nanostructures using multiple semiconductor fabrication techniques [154]. Relative to conventional boron-doped diamond electrodes, the nanostructured electrodes offer higher sensitivity to neural activity while maintaining stability and biocompatibility, suggesting that these electrodes can be applied in the development of highly sensitive BMI systems and rehabilitation instruments.
CONCLUSIONS
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
In this report, we have reviewed the application of current multi-channel neural probes (i.e., the tetrode, Utah array, and Michigan probe) to BMI systems, as well as developments regarding next-generation neural probes. When comparing the electrodes currently in use, it is important to choose the proper neural probe based on the function and purpose of the BMI application. For instance, for measurement and stimulation of pyramidal neurons in deep sulcus areas—which are known to directly control fine muscles such as those in the hand—a neural probe with a longer shank is required. We also discussed several developing neural probes, which aim to improve upon the electrical properties, biocompatibility, and reliability of current devices using multi-modal approaches. If high-density electrodes with improved biocompatibility can be developed, flexible ECoG systems can be more widely applied in both clinical and preclinical BMI studies. Although stent electrodes are currently at the proof-of-concept stage, their minimal invasiveness suggests that they can replace many other methods for acquiring neural signals in the future. Safety concerns represent the greatest challenge for BMI systems based on optogenetic stimulation. The application of new materials, high-resolution integrated technologies, and nanotechnology will ultimately contribute to the development of high-performance neural probes and BMI systems.
While the long-term fidelity of neural recordings is critical for success in BMI systems, several subsequent stages of processing are critical for ensuring the efficiency of these systems (Fig. 1). Decoding algorithms that allow for accurate analysis of acquired neural signals and encoding techniques that transfer outward information to the brain are also important components of a BMI system [155,156,157,158]. High-speed computing and wireless signal processing are also critical for the development of high-performance BMI systems that can be applied in real-world settings.
Perhaps the most important issue facing developers is the biocompatibility and mechanical suitability of implantable neural probes. Advancements in material science and mechanical techniques will aid in developing strategies for minimizing brain scarring while maintaining adequate electrode contact [159,160]. Assessments of signal quality and side effects should be performed in both
Mass production and security concerns should also be addressed in the development of neural probes for commercial and real-world use. Because the electrode is inserted into the brain, proper mass manufacturing technologies are required, without sacrificing the quality of the probe from production to packaging. Thus, systematic inspection methods should also develop. Several security experts have cautioned that information leakage may occur when using BMI systems [161, 162]. Therefore, establishing technologies to prevent information leakage is another issue that must be addressed when considering the practical applications of BMI systems. In conjunction with technological advancements, consensus regarding social and ethical concerns will lead to widespread utilization of implantable neural probes and BMI systems.
Figures
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference

References
- Go to
- Abstract
- Graphical Abstract
- INTRODUCTION
- APPLICATION OF IMPLANTABLE NEURAL PROBES IN BMIs
- TETRODE
- UTAH ARRAY
- MICHIGAN PROBE
- RECENT ADVANCES IN NEURAL PROBES FOR IMPROVEMENTS OF BMI SYSTEMS
- NOVEL IMPLANTABLE NEURAL PROBES WITH DECREASING A DEGREE OF INVASIVENESS AND MAINTAINING PERFORMANCE
- MULTI-MODAL NEURAL PROBES TO MEASURE BOTH ELECTRICAL AND OPTICAL SIGNALS
- NEURAL PROBES WITH ADVANCED MATERIALS
- CONCLUSIONS
- Figure
- Reference
- Nair P. Brain-machine interface. Proc Natl Acad Sci U S A 2013;110:18343.
- Fetz EE. Restoring motor function with bidirectional neural interfaces. Prog Brain Res 2015;218:241-252.
- Merritt B. In: Iniewski K. Synthesis lectures on emerging engineering technologies. San Rafael, CA: Morgan & Claypool Publishers, 2016; 2016. p. 1-109.
- Patil PG, Turner DA. The development of brain-machine interface neuroprosthetic devices. Neurotherapeutics 2008;5:137-146.
- Bell CJ, Shenoy P, Chalodhorn R, Rao RP. Control of a humanoid robot by a noninvasive brain-computer interface in humans. J Neural Eng 2008;5:214-220.
- Kansaku K, Hata N, Takano K. My thoughts through a robot's eyes: an augmented reality-brain-machine interface. Neurosci Res 2010;66:219-222.
- Miranda RA, Casebeer WD, Hein AM, Judy JW, Krotkov EP, Laabs TL, Manzo JE, Pankratz KG, Pratt GA, Sanchez JC, Weber DJ, Wheeler TL, Ling GS. DARPA-funded efforts in the development of novel brain-computer interface technologies. J Neurosci Methods 2015;244:52-67.
- Panzeri S, Safaai H, De Feo V, Vato A. Implications of the dependence of neuronal activity on neural network states for the design of brain-machine interfaces. Front Neurosci 2016;10:165.
- Millán Jdel R, Renkens F, Mouriño J, Gerstner W. Noninvasive brain-actuated control of a mobile robot by human EEG. IEEE Trans Biomed Eng 2004;51:1026-1033.
- Rebsamen B, Guan C, Zhang H, Wang C, Teo C, Ang MH, Burdet E. A brain controlled wheelchair to navigate in familiar environments. IEEE Trans Neural Syst Rehabil Eng 2010;18:590-598.
- Müller KR, Tangermann M, Dornhege G, Krauledat M, Curio G, Blankertz B. Machine learning for real-time single-trial EEG-analysis: from brain-computer interfacing to mental state monitoring. J Neurosci Methods 2008;167:82-90.
- Fazli S, Mehnert J, Steinbrink J, Curio G, Villringer A, Müller KR, Blankertz B. Enhanced performance by a hybrid NIRS-EEG brain computer interface. Neuroimage 2012;59:519-529.
- Coyle SM, Ward TE, Markham CM. Brain-computer interface using a simplified functional near-infrared spectroscopy system. J Neural Eng 2007;4:219-226.
- Kauhanen L, Nykopp T, Lehtonen J, Jylänki P, Heikkonen J, Rantanen P, Alaranta H, Sams M. EEG and MEG brain-computer interface for tetraplegic patients. IEEE Trans Neural Syst Rehabil Eng 2006;14:190-193.
- Yeom HG, Kim JS, Chung CK. Estimation of the velocity and trajectory of three-dimensional reaching movements from non-invasive magnetoencephalography signals. J Neural Eng 2013;10:026006.
- Iturrate I, Antelis JM, Kubler A, Minguez J. A noninvasive brain-actuated wheelchair based on a P300 neurophysiological protocol and automated navigation. IEEE Trans Robot 2009;25:614-627.
- Waldert S. Invasive vs. non-invasive neuronal signals for brain-machine interfaces: will one prevail?. Front Neurosci 2016;10:295.
- Wodlinger B, Downey JE, Tyler-Kabara EC, Schwartz AB, Boninger ML, Collinger JL. Ten-dimensional anthropomorphic arm control in a human brain-machine interface: difficulties, solutions, and limitations. J Neural Eng 2015;12:016011.
- Baranauskas G. What limits the performance of current invasive brain machine interfaces?. Front Syst Neurosci 2014;8:68.
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science 1986;233:1416-1419.
- Adrian ED, Bronk DW. The discharge of impulses in motor nerve fibres: part II. The frequency of discharge in reflex and voluntary contractions. J Physiol 1929;67:i3-i151.
- Ling G, Gerard RW. The normal membrane potential of frog sartorius fibers. J Cell Comp Physiol 1949;34:383-396.
- Bretag AH. The glass micropipette electrode: a history of its inventors and users to 1950. J Gen Physiol 2017;149:417-430.
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsáki G. Intracellular features predicted by extracellular recordings in the hippocampus
in vivo . J Neurophysiol 2000;84:390-400. - Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol 2003;551:139-153.
- Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci 2004;7:446-451.
- Takahashi S, Anzai Y, Sakurai Y. A new approach to spike sorting for multi-neuronal activities recorded with a tetrode--how ICA can be practical. Neurosci Res 2003;46:265-272.
- Gray CM, Maldonado PE, Wilson M, McNaughton B. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J Neurosci Methods 1995;63:43-54.
- Shoham S, Fellows MR, Normann RA. Robust, automatic spike sorting using mixtures of multivariate t-distributions. J Neurosci Methods 2003;127:111-122.
- Nguyen DP, Layton SP, Hale G, Gomperts SN, Davidson TJ, Kloosterman F, Wilson MA. Micro-drive array for chronic
in vivo recording: tetrode assembly. J Vis Exp 2009;26:1098. - Xie K, Fox GE, Liu J, Tsien JZ. 512-channel and 13-region simultaneous recordings coupled with optogenetic manipulation in freely behaving mice. Front Syst Neurosci 2016;10:48.
- Giszter SF, Hart CB, Udoekwere UI, Markin S, Barbe C. A real-time system for small animal neurorobotics at spinal or cortical levels. Int IEEE EMBS Conf Neural Eng 2005;2005:450-453.
- Song W, Ramakrishnan A, Udoekwere UI, Giszter SF. Multiple types of movement-related information encoded in hindlimb/trunk cortex in rats and potentially available for brain-machine interface controls. IEEE Trans Biomed Eng 2009;56:2712-2716.
- Song W, Giszter SF. Adaptation to a cortex-controlled robot attached at the pelvis and engaged during locomotion in rats. J Neurosci 2011;31:3110-3128.
- Bender JA, Pollack AJ, Ritzmann RE. Neural activity in the central complex of the insect brain is linked to locomotor changes. Curr Biol 2010;20:921-926.
- Oweiss KG. A systems approach for data compression and latency reduction in cortically controlled brain machine interfaces. IEEE Trans Biomed Eng 2006;53:1364-1377.
- Kubo T, Katayama N, Karashima A, Nakao M. The 3D position estimation of neurons in the hippocampus based on the multi-site multi-unit recordings with silicon tetrodes. Conf Proc IEEE Eng Med Biol Soc 2008;2008:5021-5024.
- Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE Trans Biomed Eng 1991;38:758-768.
- Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature 2008;453:1098-1101.
- Chase SM, Kass RE, Schwartz AB. Behavioral and neural correlates of visuomotor adaptation observed through a brain-computer interface in primary motor cortex. J Neurophysiol 2012;108:624-644.
- Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, Ko WK, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, Courtine G. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016;539:284-288.
- Tabot GA, Dammann JF, Berg JA, Tenore FV, Boback JL, Vogelstein RJ, Bensmaia SJ. Restoring the sense of touch with a prosthetic hand through a brain interface. Proc Natl Acad Sci U S A 2013;110:18279-18284.
- Kim S, Callier T, Tabot GA, Gaunt RA, Tenore FV, Bensmaia SJ. Behavioral assessment of sensitivity to intracortical microstimulation of primate somatosensory cortex. Proc Natl Acad Sci U S A 2015;112:15202-15207.
- Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans Neural Syst Rehabil Eng 2005;13:524-541.
- Simeral JD, Kim SP, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng 2011;8:025027.
- Pandarinath C, Gilja V, Blabe CH, Nuyujukian P, Sarma AA, Sorice BL, Eskandar EN, Hochberg LR, Henderson JM, Shenoy KV. Neural population dynamics in human motor cortex during movements in people with ALS. Elife 2015;4:e07436.
- Gilja V, Pandarinath C, Blabe CH, Nuyujukian P, Simeral JD, Sarma AA, Sorice BL, Perge JA, Jarosiewicz B, Hochberg LR, Shenoy KV, Henderson JM. Clinical translation of a high-performance neural prosthesis. Nat Med 2015;21:1142-1145.
- Jarosiewicz B, Sarma AA, Bacher D, Masse NY, Simeral JD, Sorice B, Oakley EM, Blabe C, Pandarinath C, Gilja V, Cash SS, Eskandar EN, Friehs G, Henderson JM, Shenoy KV, Donoghue JP, Hochberg LR. Virtual typing by people with tetraplegia using a self-calibrating intracortical brain-computer interface. Sci Transl Med 2015;7:313ra179.
- Wodlinger B, Downey JE, Tyler-Kabara EC, Schwartz AB, Boninger ML, Collinger JL. Ten-dimensional anthropomorphic arm control in a human brain-machine interface: difficulties, solutions, and limitations. J Neural Eng 2015;12:016011.
- Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, Sharma G, Sederberg PB, Glenn BC, Mysiw WJ, Morgan AG, Deogaonkar M, Rezai AR. Restoring cortical control of functional movement in a human with quadriplegia. Nature 2016;533:247-250.
- Ajiboye AB, Willett FR, Young DR, Memberg WD, Murphy BA, Miller JP, Walter BL, Sweet JA, Hoyen HA, Keith MW, Peckham PH, Simeral JD, Donoghue JP, Hochberg LR, Kirsch RF. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet 2017;389:1821-1830.
- Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-Kabara EC, Bensmaia SJ, Schwartz AB, Boninger ML, Gaunt RA. Intracortical microstimulation of human somatosensory cortex. Sci Transl Med 2016;8:361ra141.
- Normann RA. Technology insight: future neuroprosthetic therapies for disorders of the nervous system. Nat Clin Pract Neurol 2007;3:444-452.
- Wark HA, Sharma R, Mathews KS, Fernandez E, Yoo J, Christensen B, Tresco P, Rieth L, Solzbacher F, Normann RA, Tathireddy P. A new high-density (25 electrodes/mm2) penetrating microelectrode array for recording and stimulating sub-millimeter neuroanatomical structures. J Neural Eng 2013;10:045003.
- Pochay P, Wise KD, Allard LF, Rutledge LT. A multichannel depth probe fabricated using electron-beam lithography. IEEE Trans Biomed Eng 1979;26:199-206.
- Vetter RJ, Otto KJ, Marzullo TC, Kipke DR. Brain-machine interfaces in rat motor cortex: neuronal operant conditioning to perform a sensory detection task. Int IEEE EMBS Conf Neural Eng 2003;2003:637-640.
- Kipke DR, Vetter RJ, Williams JC, Hetke JF. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans Neural Syst Rehabil Eng 2003;11:151-155.
- Vetter RJ, Williams JC, Hetke JF, Nunamaker EA, Kipke DR. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans Biomed Eng 2004;51:896-904.
- Frost SB, Dunham CL, Barbay S, Krizsan-Agbas D, Winter MK, Guggenmos DJ, Nudo RJ. Output properties of the cortical hindlimb motor area in spinal cord-injured rats. J Neurotrauma 2015;32:1666-1673.
- Guggenmos DJ, Azin M, Barbay S, Mahnken JD, Dunham C, Mohseni P, Nudo RJ. Restoration of function after brain damage using a neural prosthesis. Proc Natl Acad Sci U S A 2013;110:21177-21182.
- Barz F, Livi A, Lanzilotto M, Maranesi M, Bonini L, Paul O, Ruther P. Versatile, modular 3D microelectrode arrays for neuronal ensemble recordings: from design to fabrication, assembly, and functional validation in non-human primates. J Neural Eng 2017;14:036010.
- Schalk G, Leuthardt EC. Brain-computer interfaces using electrocorticographic signals. IEEE Rev Biomed Eng 2011;4:140-154.
- Pistohl T, Ball T, Schulze-Bonhage A, Aertsen A, Mehring C. Prediction of arm movement trajectories from ECoG-recordings in humans. J Neurosci Methods 2008;167:105-114.
- Chao ZC, Nagasaka Y, Fujii N. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkeys. Front Neuroeng 2010;3:3.
- Rouse AG, Williams JJ, Wheeler JJ, Moran DW. Spatial co-adaptation of cortical control columns in a micro-ECoG brain-computer interface. J Neural Eng 2016;13:056018.
- Ryun S, Kim JS, Lee H, Chung CK. Tactile frequency-specific high-gamma activities in human primary and secondary somatosensory cortices. Sci Rep 2017;7:15442.
- Wang W, Degenhart AD, Collinger JL, Vinjamuri R, Sudre GP, Adelson PD, Holder DL, Leuthardt EC, Moran DW, Boninger ML, Schwartz AB, Crammond DJ, Tyler-Kabara EC, Weber DJ. Human motor cortical activity recorded with Micro-ECoG electrodes, during individual finger movements. Conf Proc IEEE Eng Med Biol Soc 2009;2009:586-589.
- Wang W, Collinger JL, Degenhart AD, Tyler-Kabara EC, Schwartz AB, Moran DW, Weber DJ, Wodlinger B, Vinjamuri RK, Ashmore RC, Kelly JW, Boninger ML. An electrocorticographic brain interface in an individual with Tetraplegia. PLoS One 2013;8:e55344.
- Shin D, Watanabe H, Kambara H, Nambu A, Isa T, Nishimura Y, Koike Y. Prediction of muscle activities from electrocorticograms in primary motor cortex of primates. PLoS One 2012;7:e47992.
- Rubehn B, Bosman C, Oostenveld R, Fries P, Stieglitz T. A MEMS-based flexible multichannel ECoG-electrode array. J Neural Eng 2009;6:036003.
- Henle C, Raab M, Cordeiro JG, Doostkam S, Schulze-Bonhage A, Stieglitz T, Rickert J. First long term
in vivo study on subdurally implanted micro-ECoG electrodes, manufactured with a novel laser technology. Biomed Microdevices 2011;13:59-68. - Toda H, Suzuki T, Sawahata H, Majima K, Kamitani Y, Hasegawa I. Simultaneous recording of ECoG and intracortical neuronal activity using a flexible multichannel electrode-mesh in visual cortex. Neuroimage 2011;54:203-212.
- Slutzky MW, Jordan LR, Krieg T, Chen M, Mogul DJ, Miller LE. Optimal spacing of surface electrode arrays for brain-machine interface applications. J Neural Eng 2010;7:26004.
- Tolstosheeva E, Gordillo-González V, Hertzberg T, Kempen L, Michels I, Kreiter A, Lang W. A novel flex-rigid and soft-release ECoG array. Conf Proc IEEE Eng Med Biol Soc 2011;2011:2973-2976.
- Tolstosheeva E, Gordillo-González V, Biefeld V, Kempen L, Mandon S, Kreiter AK, Lang W. A multi-channel, flex-rigid ECoG microelectrode array for visual cortical interfacing. Sensors Basel 2015;15:832-854.
- Charvet G, Foerster M, Chatalic G, Michea A, Porcherot J, Bonnet S, Filipe S, Audebert P, Robinet S, Josselin V, Reverdy J, D'Errico R, Sauter F, Mestais C, Benabid AL. A wireless 64-channel ECoG recording electronic for implantable monitoring and BCI applications: WIMAGINE. Conf Proc IEEE Eng Med Biol Soc 2012;2012:783-786.
- Mollazadeh M, Greenwald E, Thakor NV, Schieber M, Cauwenberghs G. Wireless micro-ECoG recording in primates during reach-to-grasp movements. IEEE Biomed Circuits Syst Conf 2011;2011:237-240.
- Chang CW, Chiou JC. A wireless and batteryless microsystem with implantable grid electrode/3-dimensional probe array for ECoG and extracellular neural recording in rats. Sensors Basel 2013;13:4624-4639.
- Flint RD, Wright ZA, Scheid MR, Slutzky MW. Long term, stable brain machine interface performance using local field potentials and multiunit spikes. J Neural Eng 2013;10:056005.
- Henle C, Raab M, Cordeiro JG, Doostkam S, Schulze-Bonhage A, Stieglitz T, Rickert J. First long term
in vivo study on subdurally implanted micro-ECoG electrodes, manufactured with a novel laser technology. Biomed Microdevices 2011;13:59-68. - Liu J, Fu TM, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM. Syringe-injectable electronics. Nat Nanotechnol 2015;10:629-636.
- Tian B, Liu J, Dvir T, Jin L, Tsui JH, Qing Q, Suo Z, Langer R, Kohane DS, Lieber CM. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat Mater 2012;11:986-994.
- Liu J, Xie C, Dai X, Jin L, Zhou W, Lieber CM. Multifunctional three-dimensional macroporous nanoelectronic networks for smart materials. Proc Natl Acad Sci U S A 2013;110:6694-6699.
- Patolsky F, Zheng G, Lieber CM. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat Protoc 2006;1:1711-1724.
- Javey A, Nam S, Friedman RS, Yan H, Lieber CM. Layer-by-layer assembly of nanowires for three-dimensional, multifunctional electronics. Nano Lett 2007;7:773-777.
- Fu TM, Hong G, Zhou T, Schuhmann TG, Viveros RD, Lieber CM. Stable long-term chronic brain mapping at the single-neuron level. Nat Methods 2016;13:875-882.
- Cakulev I, Efimov IR, Waldo AL. Cardioversion: past, present, and future. Circulation 2009;120:1623-1632.
- Roubin GS, Cannon AD, Agrawal SK, Macander PJ, Dean LS, Baxley WA, Breland J. Intracoronary stenting for acute and threatened closure complicating percutaneous transluminal coronary angioplasty. Circulation 1992;85:916-927.
- Jiang WJ, Wang YJ, Du B, Wang SX, Wang GH, Jin M, Dai JP. Stenting of symptomatic M1 stenosis of middle cerebral artery: an initial experience of 40 patients. Stroke 2004;35:1375-1380.
- Oxley TJ, Opie NL, John SE, Rind GS, Ronayne SM, Wheeler TL, Judy JW, McDonald AJ, Dornom A, Lovell TJ, Steward C, Garrett DJ, Moffat BA, Lui EH, Yassi N, Campbell BC, Wong YT, Fox KE, Nurse ES, Bennett IE, Bauquier SH, Liyanage KA, van der, Perucca P, Ahnood A, Gill KP, Yan B, Churilov L, French CR, Desmond PM, Horne MK, Kiers L, Prawer S, Davis SM, Burkitt AN, Mitchell PJ, Grayden DB, May CN, O'Brien TJ. Minimally invasive endovascular stent-electrode array for high-fidelity, chronic recordings of cortical neural activity. Nat Biotechnol 2016;34:320-327.
- Opie NL, van der Nagel NR, John SE, Vessey K, Rind GS, Ronayne SM, Fletcher EL, May CN, OBrien TJ, Oxley TJ. Micro-CT and histological evaluation of an neural interface implanted within a blood vessel. IEEE Trans Biomed Eng 2017;64:928-934.
- Sefcik RK, Opie NL, John SE, Kellner CP, Mocco J, Oxley TJ. The evolution of endovascular electroencephalography: historical perspective and future applications. Neurosurg Focus 2016;40:E7.
- Opie NL, John SE, Rind GS, Ronayne SM, Grayden DB, Burkitt AN, May CN, O'Brien TJ, Oxley TJ. Chronic impedance spectroscopy of an endovascular stent-electrode array. J Neural Eng 2016;13:046020.
- Schwarz DA, Lebedev MA, Hanson TL, Dimitrov DF, Lehew G, Meloy J, Rajangam S, Subramanian V, Ifft PJ, Li Z, Ramakrishnan A, Tate A, Zhuang KZ, Nicolelis MA. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat Methods 2014;11:670-676.
- Rajangam S, Tseng PH, Yin A, Lehew G, Schwarz D, Lebedev MA, Nicolelis MA. Wireless cortical brain-machine interface for whole-body navigation in primates. Sci Rep 2016;6:22170.
- Su Y, Routhu S, Moon KS, Lee SQ, Youm W, Ozturk Y. A wireless 32-channel implantable bidirectional brain machine interface. Sensors (Basel) 2016;16:E1582.
- Seo D, Carmena JM, Rabaey JM, Maharbiz MM, Alon E. Model validation of untethered, ultrasonic neural dust motes for cortical recording. J Neurosci Methods 2015;244:114-122.
- Song YK, Borton DA, Park S, Patterson WR, Bull CW, Laiwalla F, Mislow J, Simeral JD, Donoghue JP, Nurmikko AV. Active microelectronic neurosensor arrays for implantable brain communication interfaces. IEEE Trans Neural Syst Rehabil Eng 2009;17:339-345.
- Bashirullah R, Harris JG, Sanchez JC, Nishida T, Principe JC. Florida wireless implantable recording electrodes (FWIRE) for brain machine interfaces. IEEE Int Symp Circuits Syst Proc 2007;2007:2084-2087.
- Azin M, Guggenmos DJ, Barbay S, Nudo RJ, Mohseni P. A battery-powered activity-dependent intracortical microstimulation IC for brain-machine-brain interface. IEEE J Solid-State Circui. s 2011;46:731-745.
- Takahara Y, Matsuki N, Ikegaya Y. Nipkow confocal imaging from deep brain tissues. J Integr Neurosci 2011;10:121-129.
- Martial FP, Hartell NA. Programmable illumination and high-speed, multi-wavelength, confocal microscopy using a digital micromirror. PLoS One 2012;7:e43942.
- Warden MR, Cardin JA, Deisseroth K. Optical neural interfaces. Annu Rev Biomed Eng 2014;16:103-129.
- Baubet V, Le Mouellic H, Campbell AK, Lucas-Meunier E, Fossier P, Brúlet P. Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc Natl Acad Sci U S A 2000;97:7260-7265.
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 2001;32:723-735.
- Williams DA, Fogarty KE, Tsien RY, Fay FS. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature 1985;318:558-561.
- Mank M, Reiff DF, Heim N, Friedrich MW, Borst A, Griesbeck O. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys J 2006;90:1790-1796.
- Horikawa K, Yamada Y, Matsuda T, Kobayashi K, Hashimoto M, Matsu-ura T, Miyawaki A, Michikawa T, Mikoshiba K, Nagai T. Spontaneous network activity visualized by ultrasensitive Ca(2+) indicators, yellow Cameleon-Nano. Nat Methods 2010;7:729-732.
- Slovin H, Arieli A, Hildesheim R, Grinvald A. Long-term voltage-sensitive dye imaging reveals cortical dynamics in behaving monkeys. J Neurophysiol 2002;88:3421-3438.
- Petersen CC, Grinvald A, Sakmann B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured
in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci 2003;23:1298-1309. - Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron 2006;50:617-629.
- Murayama M, Larkum ME.
In vivo dendritic calcium imaging with a fiberoptic periscope system. Nat Protoc 2009;4:1551-1559. - Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. Miniaturized integration of a fluorescence microscope. Nat Methods 2011;8:871-878.
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A 2003;100:13940-13945.
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 2006;3:785-792.
- Wong J, Abilez OJ, Kuhl E. Computational optogenetics: a novel continuum framework for the photoelectrochemistry of living systems. J Mech Phys Solids 2012;60:1158-1178.
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface:
in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 2007;4:S143-S156. - Wentz CT, Bernstein JG, Monahan P, Guerra A, Rodriguez A, Boyden ES. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. J Neural Eng 2011;8:046021.
- Iwai Y, Honda S, Ozeki H, Hashimoto M, Hirase H. A simple head-mountable LED device for chronic stimulation of optogenetic molecules in freely moving mice. Neurosci Res 2011;70:124-127.
- Dagnew R, Lin YY, Agatep J, Cheng M, Jann A, Quach V, Monroe M, Singh G, Minasyan A, Hakimian J, Kee T, Cushman J, Walwyn W. CerebraLux: a low-cost, open-source, wireless probe for optogenetic stimulation. Neurophotonics 2017;4:045001.
- Yashiro H, Nakahara I, Funabiki K, Riquimaroux H. Micro-endoscopic system for functional assessment of neural circuits in deep brain regions: simultaneous optical and electrical recordings of auditory responses in mouse's inferior colliculus. Neurosci Res 2017;119:61-69.
- Zhao Z, Luan L, Wei X, Zhu H, Li X, Lin S, Siegel JJ, Chitwood RA, Xie C. Nanoelectronic coating enabled versatile multifunctional neural probes. Nano Lett 2017;17:4588-4595.
- LeChasseur Y, Dufour S, Lavertu G, Bories C, Deschênes M, Vallée R, De Koninck Y. A microprobe for parallel optical and electrical recordings from single neurons
in vivo . Nat Methods 2011;8:319-325. - Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci 2011;15:163-170.
- Voigts J, Siegle JH, Pritchett DL, Moore CI. The flex-Drive: an ultra-light implant for optical control and highly parallel chronic recording of neuronal ensembles in freely moving mice. Front Syst Neurosci 2013;7:8.
- Kwon KY, Sirowatka B, Weber A, Li W. Opto-μECoG array: a hybrid neural interface with transparent μECoG electrode array and integrated LEDs for optogenetics. IEEE Trans Biomed Circuits Syst 2013;7:593-600.
- Wang J, Wagner F, Borton DA, Zhang J, Ozden I, Burwell RD, Nurmikko AV, van Wagenen R, Diester I, Deisseroth K. Integrated device for combined optical neuromodulation and electrical recording for chronic
in vivo applications. J Neural Eng 2012;9:016001. - Boutte RW, Merlin S, Yona G, Griffiths B, Angelucci A, Kahn I, Shoham S, Blair S. Utah optrode array customization using stereotactic brain atlases and 3-D CAD modeling for optogenetic neocortical interrogation in small rodents and nonhuman primates. Neurophotonics 2017;4:041502.
- Pashaie R, Anikeeva P, Lee JH, Prakash R, Yizhar O, Prigge M, Chander D, Richner TJ, Williams J. Optogenetic brain interfaces. IEEE Rev Biomed Eng 2014;7:3-30.
- Baumgartner RA. An optogenetic brain-machine interface for spatiotemporal neuromodulation. Milwaukee, WI: The University of Wisconsin-Milwaukee, [dissertation]
- Pashaie R, Baumgartner R, Richner TJ, Brodnick SK, Azimipour M, Eliceiri KW, Williams JC. Closed-loop optogenetic brain interface. IEEE Trans Biomed Eng 2015;62:2327-2337.
- Iseri E, Kuzum D. Implantable optoelectronic probes for
in vivo optogenetics. J Neural Eng 2017;14:031001. - Ramezani R, Liu Y, Dehkhoda F, Soltan A, Haci D, Zhao H, Firfilionis D, Hazra A, Cunningham MO, Jackson A, Constandinou TG, Degenaar P. On-probe neural interface ASIC for combined electrical recording and optogenetic stimulation. IEEE Trans Biomed Circuits Syst 2018;12:576-588.
- Jia Y, Khan W, Lee B, Fan B, Madi F, Weber A, Li W, Ghovanloo M. Wireless opto-electro neural interface for experiments with small freely behaving animals. J Neural Eng 2018;15:046032.
- Mendrela AE, Kim K, English D, McKenzie S, Seymour JP, Buzsáki G, Yoon E. A high-resolution opto-electrophysiology system with a miniature integrated headstage. IEEE Trans Biomed Circuits Syst 2018;99:1-11.
- Liu X, Lu Y, Iseri E, Shi Y, Kuzum D. A compact closed-loop optogenetics system based on artifact-free transparent graphene electrodes. Front Neurosci 2018;12:132.
- Chow BY, Boyden ES. Optogenetics and translational medicine. Sci Transl Med 2013;5:177ps5.
- Gaub BM, Berry MH, Visel M, Holt A, Isacoff EY, Flannery JG. In: Boon CJF, Wijnholds J. Retinal gene therapy. New York, NY: Humana Press, 2018; 2018. p. 177-189.
- Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes--the route toward applications. Science 2002;297:787-792.
- Coleman JN, Khan U, Blau WJ, Gun'ko YK. Small but strong: a review of the mechanical properties of carbon nanotube-polymer composites. Carbon 2006;44:1624-1652.
- Wang K, Fishman HA, Dai H, Harris JS. Neural stimulation with a carbon nanotube microelectrode array. Nano Lett 2006;6:2043-2048.
- Keefer EW, Botterman BR, Romero MI, Rossi AF, Gross GW. Carbon nanotube coating improves neuronal recordings. Nat Nanotechnol 2008;3:434-439.
- Guitchounts G, Markowitz JE, Liberti WA, Gardner TJ. A carbon-fiber electrode array for long-term neural recording. J Neural Eng 2013;10:046016.
- Vitale F, Summerson SR, Aazhang B, Kemere C, Pasquali M. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano 2015;9:4465-4474.
- Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS. Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 2010;22:3906-3924.
- Chen CH, Lin CT, Hsu WL, Chang YC, Yeh SR, Li LJ, Yao DJ. A flexible hydrophilic-modified graphene microprobe for neural and cardiac recording. Nanomedicine (Lond) 2013;9:600-604.
- Park DW, Schendel AA, Mikael S, Brodnick SK, Richner TJ, Ness JP, Hayat MR, Atry F, Frye ST, Pashaie R, Thongpang S, Ma Z, Williams JC. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat Commun 2014;5:5258.
- Kuzum D, Takano H, Shim E, Reed JC, Juul H, Richardson AG, de Vries J, Bink H, Dichter MA, Lucas TH, Coulter DA, Cubukcu E, Litt B. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat Commun 2014;5:5259.
- Liu TC, Chuang MC, Chu CY, Huang WC, Lai HY, Wang CT, Chu WL, Chen SY, Chen YY. Implantable graphene-based neural electrode interfaces for electrophysiology and neurochemistry in
in vivo hyperacute stroke model. ACS Appl Mater Interfaces 2016;8:187-196. - Apollo NV, Maturana MI, Tong W, Nayagam DAX, Shivdasani MN, Foroughi J, Wallace GG, Prawer S, Ibbotson MR, Garrett DJ. Soft, flexible freestanding neural stimulation and recording electrodes fabricated from reduced graphene oxide. Adv Funct Mater 2015;25:3551-3559.
- Park S, Song YJ, Boo H, Chung TD. Nanoporous Pt microelectrode for neural stimulation and recording:
in vitro characterization. J Phys Chem C 2010;114:8721-8726. - Suyatin DB, Wallman L, Thelin J, Prinz CN, Jörntell H, Samuelson L, Montelius L, Schouenborg J. Nanowire-based electrode for acute
in vivo neural recordings in the brain. PLoS One 2013;8:e56673. - Abidian MR, Corey JM, Kipke DR, Martin DC. Conducting-polymer nanotubes improve electrical properties, mechanical adhesion, neural attachment, and neurite outgrowth of neural electrodes. Small 2010;6:421-429.
- Piret G, Hébert C, Mazellier JP, Rousseau L, Scorsone E, Cottance M, Lissorgues G, Heuschkel MO, Picaud S, Bergonzo P. 3D-nanostructured boron-doped diamond for microelectrode array neural interfacing. Biomaterials 2015;53:173-183.
- Kim SP, Simeral JD, Hochberg LR, Donoghue JP, Black MJ. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J Neural Eng 2008;5:455-476.
- Li Z, O'Doherty JE, Lebedev MA, Nicolelis MA. Adaptive decoding for brain-machine interfaces through Bayesian parameter updates. Neural Comput 2011;23:3162-3204.
- Shoham S, Paninski LM, Fellows MR, Hatsopoulos NG, Donoghue JP, Normann RA. Statistical encoding model for a primary motor cortical brain-machine interface. IEEE Trans Biomed Eng 2005;52:1312-1322.
- Nishimoto S, Vu AT, Naselaris T, Benjamini Y, Yu B, Gallant JL. Reconstructing visual experiences from brain activity evoked by natural movies. Curr Biol 2011;21:1641-1646.
- Egert D, Najafi K. New class of chronic recording multichannel neural probes with post-implant self-deployed satellite recording sites. Int Solid State Sens Actuators Microsyst Conf 2011;2011:958-961.
- Daneshvar ED, Kipke D, Smela E. Navigating conjugated polymer actuated neural probes in a brain phantom. Proc SPIE 2012;8340:834009.
- Denning T, Matsuoka Y, Kohno T. Neurosecurity: security and privacy for neural devices. Neurosurg Focus 2009;27:E7.
- Bonaci T, Herron J, Matlack C, Chizeck HJ. Securing the exocortex: a twenty-first century cybernetics challenge. IEEE Technol Soc Mag 2015;34:44-51.



