Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2019; 28(1): 43-53
Published online January 30, 2019
https://doi.org/10.5607/en.2019.28.1.43
© The Korean Society for Brain and Neural Sciences
14-3-3γ Haploinsufficient Mice Display Hyperactive and Stress-sensitive Behaviors
Do Eon Kim1,†, Chang-Hoon Cho2,†, Kyoung Mi Sim2, Osung Kwon2, Eun Mi Hwang3, Hyung-Wook Kim1*, and Jae-Yong Park2*
1College of Life Sciences, Sejong University, Seoul 05006, Korea.
2School of Biosystem and Biomedical Science, College of Health Science, Korea University, Seoul 02708, Korea.
3Center for Functional Connectomics, Korea Institute of Science and Technology (KIST), Seoul 02792, Korea.
Correspondence to: *To whom correspondence should be addressed.
Jae-Yong Park, TEL: 82-2-3290-5637, FAX: 82-2-917-2388
e-mail: jaeyong68@korea.ac.kr
Hyung-Wook Kim, TEL: 82-2-3408-3202, FAX: 82-2-3408-4334
e-mail: kimhyung@sejong.ac.kr
†These authors contribute equally for this study
Abstract
14-3-3γ plays diverse roles in different aspects of cellular processes. Especially in the brain where 14-3-3γ is enriched, it has been reported to be involved in neurological and psychiatric diseases (e.g. Williams-Beuren syndrome and Creutzfeldt-Jakob disease). However, behavioral abnormalities related to 14-3-3γ deficiency are largely unknown. Here, by using 14-3-3γ deficient mice, we found that homozygous knockout mice were prenatally lethal, and heterozygous mice showed developmental delay relative to wild-type littermate mice. In addition, in behavioral analyses, we found that 14-3-3γ heterozygote mice display hyperactive and depressive-like behavior along with more sensitive responses to acute stress than littermate control mice. These results suggest that 14-3-3γ levels may be involved in the developmental manifestation of related neuropsychiatric diseases. In addition, 14-3-3γ heterozygote mice may be a potential model to study the molecular pathophysiology of neuropsychiatric symptoms.
Graphical Abstract
Keywords: 14-3-3γ, Ywhag, Hyperactivity, Anxiety, Acute stress, ADHD
INTRODUCTION
14-3-3 proteins are a family of ubiquitously expressed adaptor proteins that are involved in diverse cellular processes, such as intracellular signaling, cell-cycle control, apoptosis, neuronal migration, and protein trafficking by regulating hundreds of different “client” proteins [1,2,3]. 14-3-3 proteins act as a dimer by binding to phosphorylated target proteins at specific site(s), causing a conformational change [4]. The interaction of 14-3-3 proteins with specific partners affects their stability, localization, and activities within the cell [2,3,4].
Since 14-3-3 proteins are enriched in the brain, they have been reported to be involved in a broad range of brain functions, and neurological and psychiatric diseases [2,5]. 14-3-3 proteins have also been shown to be involved in axon growth and pathfinding as well as neuronal regeneration [6,7]. In addition, transgenic mice expressing difopein, a 14-3-3 peptide inhibitor, displayed impairments of hippocampus-dependent learning and memory tasks, and long-term synaptic plasticity of hippocampal synapses [8]. These mice also display schizophrenia-like behaviors [9].
The behavioral phenotypes of two 14-3-3 isoform-specific null mice have been reported [10,11]. In these studies, 14-3-3ε knockout (KO) mice displayed enhanced anxiety-like behaviors and memory deficits as well as morphological abnormalities in the hippocampus [11]. 14-3-3ζ KO mice displayed hyperactive behaviors and cognitive deficits, as well as abnormal neuronal migration in the hippocampus during development [10].
14-3-3γ, encoded by
14-3-3γ is broadly expressed in various tissues including brain, liver, heart, ovary, thymus, spleen, and placenta, and it is considered mainly as a cytosolic protein to interact with diverse “client” proteins [1,22]. According to the Allen brain atlas database (http://mouse.brain-map.org), 14-3-3γ is broadly expressed throughout the whole brain including cerebral cortex and hippocampus as previously shown [23,24]. Although 14-3-3γ has been previously shown to be expressed in neurons and astrocytes in the brain, cell-type specific expression pattern of 14-3-3γ is largely unknown [13,24,25].
Previously, we have shown that 14-3-3γ regulates neuronal differentiation and surface expression of TRPM4b, ANO1 and BEST1 [26,27,28]. In addition, previous studies also showed that both overexpression and shRNA-mediated silencing of 14-3-3γ in the embryonic mouse brain resulted in neuronal migration delay and morphological defects in the developing cerebral cortex [23,24]. Thus, the expression of 14-3-3γ in the brain may be critical for proper brain function. Although a previous report showed that there was no apparent phenotype in 14-3-3γ null mice [29], here, we examined a new 14-3-3γ null mouse generated using a gene-trap strategy [30].
MATERIALS AND METHODS
Transgenic
The whole brain tissues obtained were lysed using RIPA buffer containing a protease inhibitor cocktail (Roche) and processed for western blotting using anti-14-3-3γ antibody (sc-398423, 1:1000, Santa Cruz Biotechnology) and mouse anti-actin (1:2000; Sigma-Aldrich). Signals were detected using enhanced chemiluminescence (GE Healthcare, Chicago, IL, USA) following probing with the appropriate horseradish peroxidase-conjugated secondary antibodies (1:3000, Jackson ImmunoResearch). Each experiment was performed with samples from three independent groups.
Animals were acclimatized to the behavior test room for a week before testing. Behavioral assays were commenced when the mice were aged 10 weeks. One cohort of mice were used for all behavioral assays (n=8 per genotype). The order of behavioral tests progresses from less stressful one (e.g. open field test) to more stressful one (e.g. forced swim test) and there were two or three day's interval between assays.
The open field test (OFT) was performed as described to measure locomotor activity [31]. The open field apparatus consisted of a square arena (40×40 cm) with 30 cm high walls that was illuminated using an electric bulb hanging 2.5 m above the floor. Open field behaviors of the mice were recorded and analyzed using the ANY-maze system (Stoelting, Wood Dale, IL). Animals were exposed to the open field for 10 minutes. Between subjects, the box was thoroughly cleaned with 70% ethanol and the ethanol was allowed to evaporate completely prior to testing mice.
The elevated plus maze (EPM) test was performed as described [32]. The apparatus consisted of two open arms and two enclosed arms arranged in a plus-sign orientation. The arms were elevated 50 cm above the floor with each 5-cm wide arm projecting 30 cm from the center. At the start of the test, each subject was placed in the center of the EPM facing a closed arm. Mice explored the maze for 5 minutes and exploratory activities in both the open and closed arms were recorded and analyzed using the ANY-maze system (Stoelting, Wood Dale, IL). Between subjects, the maze was thoroughly cleaned with 70% ethanol and the ethanol was allowed to evaporate completely prior to testing mice.
This test was performed as described [33]. The apparatus consisted of two (light and dark) chambers (34×24×24 cm) joined together. There was an aperture (8×8 cm) between the two chambers that the mice could use to move between chambers. The chambers were made of white and black opaque Plexiglas. The black chamber was covered with a black lid (the dark box), while the white chamber was covered with a transparent lid and illuminated using ambient room lighting to ~450 lux (the light box). During the test, a subject was placed in the center of the light box facing away from the aperture connecting the two chambers and allowed to freely explore both chambers for 5 minutes. Between animals, the box was cleaned with 70% ethanol and allowed to completely dry. All sessions were recorded and analyzed using the ANY-maze system (Stoelting, Wood Dale, IL).
Mice were tested in a Plexiglas cage divided into three chambers comprising two equal-sized end areas (31.5×25.5 cm each) and a smaller neutral section between them (10.5×25.5 cm). During the habituation phase, the end areas contained an empty “holding cell” (10.16 cm in diameter and 13.97 cm tall). Each mouse was placed in the center of the box and allowed to explore the entire box for 5 minutes. The subject was then returned to the cage while another adult male (C57BL/6 WT mice) was placed under the holding cell (on one randomly selected side) and the empty cell was placed on the opposite side. During the test phase, the subjects were placed in the center and allowed to investigate the entire box for 5 minutes. The ANY-maze system recorded and scored the number of entrances into each side and the time spent investigating the novel mouse. Investigation was defined as the test mouse's nose touching the novel mouse through the bars or sniffing within 1 cm. The test arena was carefully cleaned with 70% ethanol between subjects and allowed to completely dry.
The rotarod test was performed to evaluate the basic mobility of animals in each group as described [31]. Mice were placed on the stationary cylinder of the rotarod apparatus and trained on the apparatus for at least four consecutive trials in which the rod was kept at a constant speed (4 rpm) and returned to their cages for 1 min between trials. Once the animals were able to stay on the rod rotating at 4 rpm for at least 60 s, they were subjected to the rotarod test. Mice were placed on the rod rotating at an accelerating speed from 4 to 40 rpm over 300 s. The time before animals fell off the rod was recorded with a maximum cut-off of 300 s. Mice were tested for eight consecutive trials with at least 5-min intervals. The data from the last four trials were averaged as the latency to fall. Each mouse was returned to its home cage and remained there for 5 min before the next trial. If the mouse remained on the rod in 5 minutes, latency to fall was recorded as 300 s. Between subjects, the rotarod apparatus was thoroughly cleaned with 70% ethanol and allowed to completely dry.
Mice were subjected to a schedule of restraint stress before each behavior test to induce behavioral and physiological phenotypes. Each mouse was restrained using a 50 ml conical tube with an open end, exposing the nose for breathing. Acute stress was applied for one hour and each mouse was returned to its home cage immediately after restraining stress.
All statistical analyses were carried out using GraphPad Prism version 5.00 for Windows. Numerical data are presented as means±standard error of the mean (SEM). The error bars in graphs denote the SEM. The statistical significance of the data was assessed using unpaired or paired Student's
RESULTS
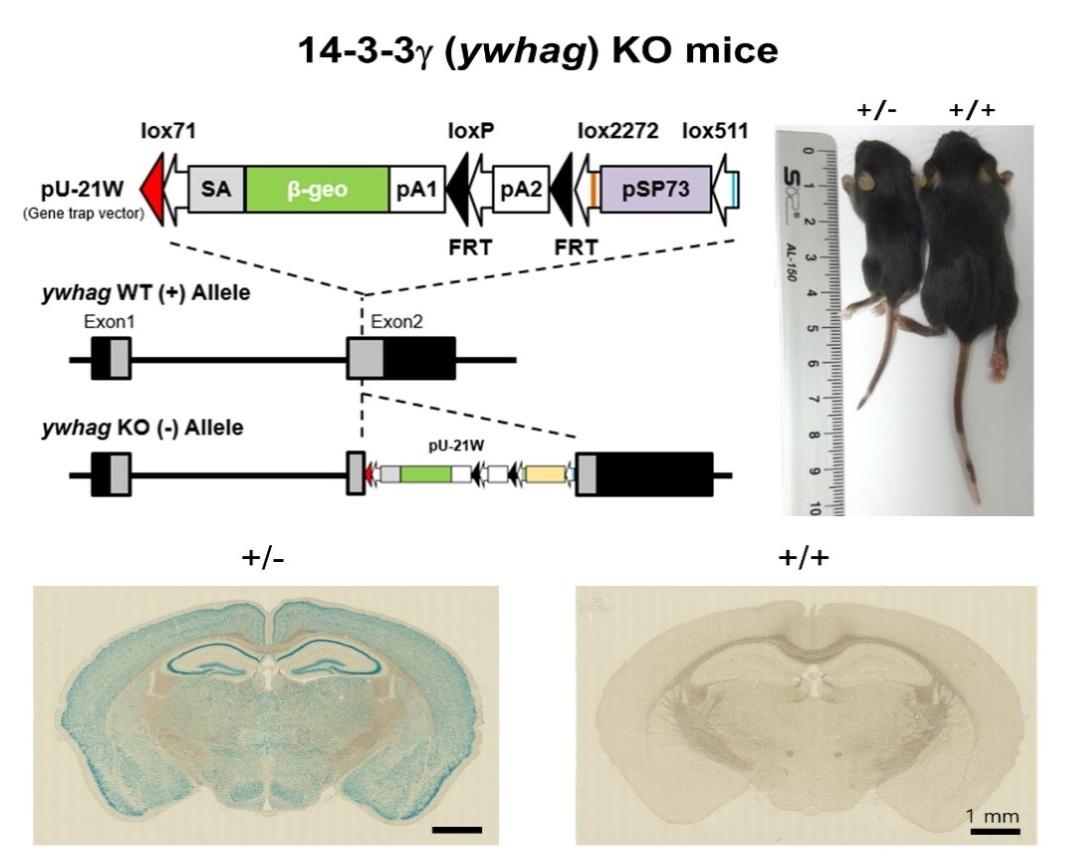
The genomic organization of the mouse 14-3-3γ gene (
To verify the genetic deletion of
Interestingly, at weaning (P21), we found that there were no surviving homozygote KO mice among the 242 mice born from 15 breeding pairs (36 litters of pups) (Fig. 3A). This is clearly distinct from the other two homozygote KO mice of 14-3-3 isoform genes (
Since 14-3-3γ is abundantly expressed in the brain, to investigate its role in the expression of behavioral characteristics, we performed an OFT to assess basal locomotor activity. Intriguingly, 14-3-3γ Het mice traveled significantly more distance in the center zone than WT mice (Fig. 4A), which may be due to hyperactivity and not related to less anxiety, because the total distance traveled and mean speed of 14-3-3γ Het mice were significantly longer and faster than those in WT mice, respectively (Fig. 4B and 4C). In addition, the Het mice also showed significant longer margin distance compared to WT mice (data not shown). Because there was no significant difference between WT and Het mice in the rotarod test, any change in muscle strength or coordination of Het mice was not related (Fig. 4D).
Since the OFT suggested that the reduced level of 14-3-3γ in Het mice results in hyperactive behavior, to determine whether the 14-3-3γ haploinsufficiency may affect mood-related behaviors, we conducted the forced swim test (FST) and three-chamber social interaction test (3CT). Interestingly, Het mice displayed significantly longer immobile time in the FST than WT mice (Fig. 5A), which suggests that their depressive-like phenotype was induced by 14-3-3γ haploinsufficiency, while there was no deficit in the social interaction of Het mice in the 3CT (Fig. 5B).
Hyperactive and depressive symptoms are common in patients with several neuropsychiatric diseases. Since these patients often have a deficit in the appropriate response to acute stress [34], we subjected Het mice to restraint stress and performed several behavioral tests including the OFT, light and dark box (LDB) test, and EPM test. In the OFT, the hyperactivity of Het mice was significantly decreased, as seen in the total distance traveled and mean speed (Fig. 6A and 6B), while WT littermate control mice did not show any significant change. In the LDB test, total distance traveled in the light zone and number of transitions were significantly increased in Het mice in response to acute stress (Fig. 6C and 6D). Similarly, Het mice showed significantly increased time spent in open ends and total distance traveled in open arms in response to acute stress in the EPM test (Fig. 6E and 6F).
DISCUSSION
14-3-3γ proteins play diverse roles in many different cellular processes [1,3,23,24]. In addition to the role as an adaptor protein facilitating protein-protein interactions in diverse cellular signaling processes, 14-3-3γ has been shown to be involved in transporting ion channels, transporters, and transmembrane receptors to the plasma membrane such as BK channels, TRPM4, ANO1, and BEST-1 in excitable and non-excitable cells [26,27,28,36]. Furthermore, 14-3-3γ has been implicated in several neurological and psychiatric diseases such as spinocerebellar ataxia type 1, Parkinson's disease, and tuberous sclerosis [15,16,17]. Especially, recent studies showed that
Unlike the potential significance of 14-3-3γ in brain development from these studies, a previous study showed that 14-3-3γ null mice display no apparent behavioral abnormalities [29]. These 14-3-3γ KO mice were generated by the genomic replacement of exon 2 with neomycin resistance cassette to delete exon 2 of 14-3-3γ [29]. Authors of this study suggested that other endogenous 14-3-3 isoforms may compensate for the loss of 14-3-3γ. In contrast, we found that 14-3-3γ homozygous KO mice were prenatally lethal, and heterozygous mice showed developmental delay relative to littermate wild-type mice (Fig. 2 and 3). In our mice, the
In behavioral experiments, we found that 14-3-3γ Het mice display hyperactive locomotor activity, and more sensitive responses to acute stress than WT mice. Hyperactive locomotor activity, observed in the OFT, was not due to any alteration in muscle strength or endurance since there was no significant difference in latency to fall in the rotarod test between WT and Het mice. 14-3-3γ Het mice showed significantly increased depressive-like behavior in the FST relative to WT mice. After exposure to acute stress, we observed that the hyperactivity feature of Het mice was significantly reduced in the OFT. On the other hand, Het mice showed increased travel distance in the light zone and number of transitions in the LDB test, as well as increased distance and time spent in the open arms in the EPM test. Based on these stress-response behaviors, reduced level of 14-3-3γ in Het mice may increase the sensitivity to exogenous stimuli relative to WT. Although this study clearly showed the significant relevance of 14-3-3γ in mouse behaviors, the correlation between cell-type specific expressions of 14-3-3γ in the brain and its related behavioral phenotypes need to be examined in detail.
Hyperactivity and hypersensitivity are frequently observed as comorbid symptoms in many neuropsychiatric diseases, such as attention deficit hyperactivity disorder (ADHD). Hyperactivity has multiple manifestations, including continuous movement, being distracted, and impulsive behaviors. ADHD patients with hypersensitivity are highly agitated when faced with various challenges including sensory stimuli and psychological stress. This condition makes individuals overactive and inattentive from unknown causes, which often prohibits individuals from living normal lives [40]. Interestingly, the behavioral phenotypes of our 14-3-3γ Het mice display two characteristics of ADHD (Fig. 4,5,6). Recently, WBS, a 14-3-3γ-related neurodevelopmental disease, has been shown to be strongly associated with ADHD with hyperactivity and hypersensitivity [41,42]. Since 14-3-3γ Het mice displayed neurodevelopmental delay, hypersensitivity and hyperactivity, we believe that our 14-3-3γ Het mice can be a good mouse model for ADHD and WBS.
There are features of 14-3-3γ distinct from the other 14-3-3 proteins although functions of 14-3-3 isoforms seem to be redundant and their expression patterns in organisms are vastly overlapping [35,43,44]. For example, the elevated level of 14-3-3γ in the brain has been considered a potential biomarker in Creutzfeldt-Jakob disease [44]. In an animal model of multiple sclerosis and ischemia, 14-3-3γ has been reported to play significant roles [35,43]. In addition, reduced expression of 14-3-3γ in zebrafish caused deficits in brain development and decreased brain size [38] and altered expression of 14-3-3γ in mice delayed neuronal migration in the cerebral cortex [23,24]. Therefore, our 14-3-3γ Het mice may also be valuable to study the pathophysiological mechanisms of these diseases.
Figures






References
- Aghazadeh Y, Papadopoulos V. The role of the 14-3-3 protein family in health, disease, and drug development. Drug Discov Today 2016;21:278-287.
- Cornell B, Toyo-Oka K. 14-3-3 proteins in brain development: neurogenesis, neuronal migration and neuromorphogenesis. Front Mol Neurosci 2017;10:318.
- Kaplan A, Morquette B, Kroner A, Leong S, Madwar C, Sanz R, Banerjee SL, Antel J, Bisson N, David S, Fournier AE. Small-molecule stabilization of 14-3-3 protein-protein interactions stimulates axon regeneration. Neuron 2017;93:1082-1093.
- Mackintosh C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J 2004;381:329-342.
- Zhang J, Zhou Y. 14-3-3 proteins in glutamatergic synapses. Neural Plast 2018;2018:8407609.
- Kent CB, Shimada T, Ferraro GB, Ritter B, Yam PT, McPherson PS, Charron F, Kennedy TE, Fournier AE. 14-3-3 proteins regulate protein kinase a activity to modulate growth cone turning responses. J Neurosci 2010;30:14059-14067.
- Yam PT, Kent CB, Morin S, Farmer WT, Alchini R, Lepelletier L, Colman DR, Tessier-Lavigne M, Fournier AE, Charron F. 14-3-3 proteins regulate a cell-intrinsic switch from sonic hedgehog-mediated commissural axon attraction to repulsion after midline crossing. Neuron 2012;76:735-749.
- Qiao H, Foote M, Graham K, Wu Y, Zhou Y. 14-3-3 proteins are required for hippocampal long-term potentiation and associative learning and memory. J Neurosci 2014;34:4801-4808.
- Foote M, Qiao H, Graham K, Wu Y, Zhou Y. Inhibition of 14-3-3 proteins leads to schizophrenia-related behavioral phenotypes and synaptic defects in mice. Biol Psychiatry 2015;78:386-395.
- Cheah PS, Ramshaw HS, Thomas PQ, Toyo-Oka K, Xu X, Martin S, Coyle P, Guthridge MA, Stomski F, van den Buuse M, Wynshaw-Boris A, Lopez AF, Schwarz QP. Neurodevelopmental and neuropsychiatric behaviour defects arise from 14-3-3ζ deficiency. Mol Psychiatry 2012;17:451-466.
- Wachi T, Cornell B, Toyo-Oka K. Complete ablation of the 14-3-3epsilon protein results in multiple defects in neuropsychiatric behaviors. Behav Brain Res 2017;319:31-36.
- Dougherty MK, Morrison DK. Unlocking the code of 14-3-3. J Cell Sci 2004;117:1875-1884.
- Lai XJ, Ye SQ, Zheng L, Li L, Liu QR, Yu SB, Pang Y, Jin S, Li Q, Yu AC, Chen XQ. Selective 14-3-3γ induction quenches p-β-catenin Ser37/Bax-enhanced cell death in cerebral cortical neurons during ischemia. Cell Death Dis 2014;5:e1184.
- Peoc'h K, Schröder HC, Laplanche J, Ramljak S, Müller WE. Determination of 14-3-3 protein levels in cerebrospinal fluid from Creutzfeldt-Jakob patients by a highly sensitive capture assay. Neurosci Lett 2001;301:167-170.
- Rosner M, Hanneder M, Siegel N, Valli A, Hengstschläger M. The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat Res 2008;658:234-246.
- Umahara T, Uchihara T, Yagishita S, Nakamura A, Tsuchiya K, Iwamoto T. Intranuclear immunolocalization of 14-3-3 protein isoforms in brains with spinocerebellar ataxia type 1. Neurosci Lett 2007;414:130-135.
- Shimada T, Fournier AE, Yamagata K. Neuroprotective function of 14-3-3 proteins in neurodegeneration. BioMed Res Int 2013;2013:564534.
- Coe BP, Stessman HA, Sulovari A, Geisheker MR, Bakken TE, Lake AM, Dougherty JD, Lein ES, Hormozdiari F, Bernier RA, Eichler EE. Neurodevelopmental disease genes implicated by
de novo mutation and copy number variation morbidity. Nat Genet 2019;51:106-116. - Fusco C, Micale L, Augello B, Teresa Pellico M, Menghini D, Alfieri P, Cristina Digilio M, Mandriani B, Carella M, Palumbo O, Vicari S, Merla G. Smaller and larger deletions of the Williams Beuren syndrome region implicate genes involved in mild facial phenotype, epilepsy and autistic traits. Eur J Hum Genet 2014;22:64-70.
- Ramocki MB, Bartnik M, Szafranski P, Kołodziejska KE, Xia Z, Bravo J, Miller GS, Rodriguez DL, Williams CA, Bader PI, Szczepanik E, Mazurczak T, Antczak-Marach D, Coldwell JG, Akman CI, McAlmon K, Cohen MP, McGrath J, Roeder E, Mueller J, Kang SH, Bacino CA, Patel A, Bocian E, Shaw CA, Cheung SW, Mazurczak T, Stankiewicz P. Recurrent distal 7q11.23 deletion including HIP1 and YWHAG identified in patients with intellectual disabilities, epilepsy, and neurobehavioral problems. Am J Hum Genet 2010;87:857-865.
- Nicita F, Garone G, Spalice A, Savasta S, Striano P, Pantaleoni C, Spartà MV, Kluger G, Capovilla G, Pruna D, Freri E, D'Arrigo S, Verrotti A. Epilepsy is a possible feature in Williams-Beuren syndrome patients harboring typical deletions of the 7q11.23 critical region. Am J Med Genet A 2016;170A:148-155.
- Watanabe M, Isobe T, Ichimura T, Kuwano R, Takahashi Y, Kondo H. Molecular cloning of rat cDNAs for beta and gamma subtypes of 14-3-3 protein and developmental changes in expression of their mRNAs in the nervous system. Brain Res Mol Brain Res 1993;17:135-146.
- Cornell B, Wachi T, Zhukarev V, Toyo-Oka K. Overexpression of the 14-3-3gamma protein in embryonic mice results in neuronal migration delay in the developing cerebral cortex. Neurosci Lett 2016;628:40-46.
- Wachi T, Cornell B, Marshall C, Zhukarev V, Baas PW, Toyooka K. Ablation of the 14-3-3gamma protein results in neuronal migration delay and morphological defects in the developing cerebral cortex. Dev Neurobiol 2016;76:600-614.
- Chen XQ, Chen JG, Zhang Y, Hsiao WW, Yu AC. 14-3-3gamma is upregulated by in vitro ischemia and binds to protein kinase Raf in primary cultures of astrocytes. Glia 2003;42:315-324.
- Cho CH, Kim E, Lee YS, Yarishkin O, Yoo JC, Park JY, Hong SG, Hwang EM. Depletion of 14-3-3γ reduces the surface expression of Transient Receptor Potential Melastatin 4b (TRPM4b) channels and attenuates TRPM4b-mediated glutamate-induced neuronal cell death. Mol Brain 2014;7:52.
- Lee YS, Lee JK, Bae Y, Lee BS, Kim E, Cho CH, Ryoo K, Yoo J, Kim CH, Yi GS, Lee SG, Lee CJ, Kang SS, Hwang EM, Park JY. Suppression of 14-3-3γ-mediated surface expression of ANO1 inhibits cancer progression of glioblastoma cells. Sci Rep 2016;6:26413.
- Oh SJ, Woo J, Lee YS, Cho M, Kim E, Cho NC, Park JY, Pae AN, Justin Lee C, Hwang EM. Direct interaction with 14-3-3γ promotes surface expression of Best1 channel in astrocyte. Mol Brain 2017;10:51.
- Steinacker P, Schwarz P, Reim K, Brechlin P, Jahn O, Kratzin H, Aitken A, Wiltfang J, Aguzzi A, Bahn E, Baxter HC, Brose N, Otto M. Unchanged survival rates of 14-3-3gamma knockout mice after inoculation with pathological prion protein. Mol Cell Biol 2005;25:1339-1346.
- Kurogi S, Sekimoto T, Funamoto T, Ota T, Nakamura S, Nagai T, Nakahara M, Yoshinobu K, Araki K, Araki M, Chosa E. Development of an efficient screening system to identify novel bone metabolism-related genes using the exchangeable gene trap mutagenesis mouse models. Sci Rep 2017;7:40692.
- Choi WS, Kim HW, Tronche F, Palmiter RD, Storm DR, Xia Z. Conditional deletion of Ndufs4 in dopaminergic neurons promotes Parkinson's disease-like non-motor symptoms without loss of dopamine neurons. Sci Rep 2017;7:44989.
- Zou J, Wang W, Pan YW, Abel GM, Storm DR, Xia Z. Conditional inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 MAP kinase is not associated with anxiety/depression-like behaviors. eNeuro 2015;2:ENEURO.0014-14.2015.
- Zou J, Storm DR, Xia Z. Conditional deletion of ERK5 MAP kinase in the nervous system impairs pheromone information processing and pheromone-evoked behaviors. PLoS One 2013;8:e76901.
- Zimmerman EC, Bellaire M, Ewing SG, Grace AA. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology 2013;38:2131-2139.
- Lee DH, Steinacker P, Seubert S, Turnescu T, Melms A, Manzel A, Otto M, Linker RA. Role of glial 14-3-3 gamma protein in autoimmune demyelination. J Neuroinflammation 2015;12:187.
- Sokolowski B, Orchard S, Harvey M, Sridhar S, Sakai Y. Conserved BK channel-protein interactions reveal signals relevant to cell death and survival. PLoS One 2011;6:e28532.
- Guella I, McKenzie MB, Evans DM, Buerki SE, Toyota EB, Van Allen MI, Suri M, Elmslie F, Simon ME, van Gassen KL, Héron D, Keren B, Nava C, Connolly MB, Demos M, Farrer MJ, Adam S, Boelman C, Bolbocean C, Candido T, Eydoux P, Horvath G, Huh L, Nelson TN, Sinclair G, van Karnebeek C, Vercauteren S, Array.
De novo mutations in YWHAG cause early-onset epilepsy. Am J Hum Genet 2017;101:300-310. - Komoike Y, Fujii K, Nishimura A, Hiraki Y, Hayashidani M, Shimojima K, Nishizawa T, Higashi K, Yasukawa K, Saitsu H, Miyake N, Mizuguchi T, Matsumoto N, Osawa M, Kohno Y, Higashinakagawa T, Yamamoto T. Zebrafish gene knockdowns imply roles for human YWHAG in infantile spasms and cardiomegaly. Genesis 2010;48:233-243.
- Aghazadeh Y, Ye X, Blonder J, Papadopoulos V. Protein modifications regulate the role of 14-3-3γ adaptor protein in cAMP-induced steroidogenesis in MA-10 Leydig cells. J Biol Chem 2014;289:26542-26553.
- Randazzo WT, Dockray S, Susman EJ. The stress response in adolescents with inattentive type ADHD symptoms. Child Psychiatry Hum Dev 2008;39:27-38.
- Rhodes SM, Riby DM, Matthews K, Coghill DR. Attention-deficit/hyperactivity disorder and Williams syndrome: shared behavioral and neuropsychological profiles. J Clin Exp Neuropsychol 2011;33:147-156.
- Uljarević M, Labuschagne I, Bobin R, Atkinson A, Hocking DR. Brief report: the impact of sensory hypersensitivity and intolerance of uncertainty on anxiety in Williams syndrome. J Autism Dev Disord 2018;48:3958-3964.
- Platholi J, Heerdt PM, Lim Tung HY, Hemmings HC. Activation of brain protein phosphatase-1(I) following cardiac arrest and resuscitation involving an interaction with 14-3-3 gamma. J Neurochem 2008;105:2029-2038.
- Humpel C, Benke T. Cerebrospinal fluid levels of 14-3-3 gamma: what does it tell us about sporadic Creutzfeldt-Jakob disease?. Pharmacology 2017;100:243-245.



