Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2019; 28(2): 183-215
Published online April 30, 2019
https://doi.org/10.5607/en.2019.28.2.183
© The Korean Society for Brain and Neural Sciences
Tweety-homolog (Ttyh ) Family Encodes the Pore-forming Subunits of the Swelling-dependent Volume-regulated Anion Channel (VRACswell) in the Brain
Young-Eun Han1,2,3, Jea Kwon1,3,4, Joungha Won1,3,5, Heeyoung An1,3,4, Minwoo Wendy Jang1,3,4, Junsung Woo3, Je Sun Lee6, Min Gu Park1,3,4, Bo-Eun Yoon7, Seung Eun Lee8, Eun Mi Hwang3, Jae-Young Jung2,3, Hyungju Park6, Soo-Jin Oh3,9, and C. Justin Lee1,2,3*
1Center for Cognition and Sociality, Institute for Basic Science, Daejeon 34126, Korea.
2Department of Neuroscience, Division of Bio-Medical Science & Technology, KIST School, Korea University of Science and Technology, Seoul 02792, Korea.
3Center for Glia-Neuron Interaction, Korea Institute of Science and Technology (KIST), Seoul 02792, Korea.
4KU-KIST, Graduate School of Converging Science and Technology, Korea University, Seoul, 02841, Korea.
5Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Korea.
6Molecular Neurobiology Laboratory, Dept. of Structure and Function of Neural Network, Korea Brain Research Institute, Daegu 41068, Korea.
7Department of molecular biology, Dankook University, Cheonan 31116, Korea.
8Virus Facility, Research Animal Resource Center, Korea Institute of Science and Technology (KIST), Seoul 02792, Korea.
9Convergence Research Center for Diagnosis, Treatment and Care System of Dementia, Korea Institute of Science and Technology (KIST), Seoul 02792, Korea.
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-42-878-9150, FAX: 82-42-878-9151, e-mail: cjl@ibs.re.kr
Abstract
In the brain, a reduction in extracellular osmolality causes water-influx and swelling, which subsequently triggers Cl−- and osmolytes-efflux via volume-regulated anion channel (VRAC). Although LRRC8 family has been recently proposed as the pore-forming VRAC which is activated by low cytoplasmic ionic strength but not by swelling, the molecular identity of the pore-forming swelling-dependent VRAC (VRACswell) remains unclear. Here we identify and characterize Tweety-homologs (TTYH1, TTYH2, TTYH3) as the major VRACswell in astrocytes. Gene-silencing of all
Graphical Abstract

Keywords: Volume-regulated anion channel, VRAC, Tweety-homolog, Ttyh, Volume regulation
INTRODUCTION
Brain volume regulation is a homeostatic process in which the ionic and osmotic balance is maintained by water movement. It is critical for proper function and health of the nervous system and is tightly controlled by a specific cell type called astrocytes due to their high and exclusive expression of the water channel, aquaporin-4 (AQP4) [1,2]. The increase of intracellular or decrease of extracellular ionic- and organic-osmolytes initiates water-influx through AQP4 and swelling, which triggers an efflux of Cl− and osmolytes via volume-regulated anion channel (VRACswell), followed by a water-efflux to restore volume. The latter process is called regulatory volume decrease (RVD) [3,4,5]. The astrocytic VRACswell has been initially characterized as an intracellular Ca2+-independent and kinase inhibitor-sensitive channel [6]. It has been subsequently shown that several organic osmolytes, notably glutamate and taurine which act as neurotransmitters, are released via the astrocytic VRACswell [7,8,9,10]. Taurine, the most abundant osmolyte has been shown to regulate the glycinergic neurotransmission in the hypothalamus [11]. Moreover, VRACswell and the released osmolytes have been implicated in several pathophysiological conditions such as cerebral edema following excessive oxidative stress, ischemia, traumatic brain injury and glioma [12,13,14,15,16]. VRACswell is also associated with many other general functions including regulation of cell cycle, apoptosis, proliferation and cellular mobility [17,18]. Reduction of VRACswell and impairment of apoptotic volume decrease have been linked to a multi-drug resistance (MDR) to chemotherapy in several cancer cells [19,20]. Although the pathophysiological functions of the astrocytic VRACswell and the released osmolytes are becoming increasingly highlighted, the precise molecular and cellular mechanisms of VRACswell activation and related functions are still poorly understood due to the lack of molecular identity of the astrocytic VRACswell.
Over the years, many Cl− channels and transmembrane proteins have been proposed as the molecular identity of VRACswell but tested with no success [17,21,22,23,24]. Since 2014, LRRC8A has been proposed as a necessary component of VRACswell in various cell lines by providing evidence of the reduction of ICl,swell with
Consistently, it has been clear that LRRC8-mediated VRACΓ and the astrocytic VRACswell do not share the same biophysical and biochemical properties. LRRC8-mediated VRACΓ in HEK293T and HeLa cells were reported to be Ca2+-dependent as evidenced by the sensitivity to both extracellular zero Ca2+ and intracellular BAPTA [30,31]. Moreover, a G protein-coupled receptor (GPCR)-mediated activation of astrocytic VRAC was also shown to be dependent on the local Ca2+ in nanodomains [32]. In contrast, the swelling-activated VRAC, which was firstly reported in human epithelial cells (intestine 407), was demonstrated to be totally independent of Ca2+ [5]. More importantly, astrocytic VRACswell was shown to be Ca2+-independent [6]. These results suggested that there exist two-types of VRACs in astrocytes: Ca2+-dependent and Ca2+-independent components of VRAC. In addition, there has been no evidence that LRRC8-mediated VRACΓ is sensitive to kinase inhibitors, which happens to be a unique property of the astrocytic VRACswell [6]. Furthermore, the glutamate permeability ratio, Pglu/PCl, of LRRC8-mediated VRACΓ was previously reported as near 0.2 [33]. In contrast, the astrocytic VRACswell has been directly shown to display a higher glutamate permeability [8]. Interestingly, the glutamate- and GABA-permeable Ca2+-activated anion channel BEST1 [34,35], but not LRRC8A, was shown to encode Aqp4-dependent VRACswell in retinal pigment epithelial cells differentiated from human-induced pluripotent stem cells (hiPSC-RPE) [31,36], suggesting that anion channels other than LRRC8A could be responsible for the astrocytic VRACswell. More importantly, mechanically-induced ATP release in astrocyte was shown to be mediated possibly by VRACswell as evidenced by the sensitivity to a known VRAC inhibitor, DCPIB [37]. However, this was not impaired by gene-silencing of LRRC8A, suggesting an involvement of other unidentified component of VRACswell [37]. Therefore, the molecular identity of the astrocytic VRACswell still remains unknown.
Previous studies reported that the maxi-chloride channels share the common physiological, biophysical and pharmacological profiles with VRACswell [38]. Human TTYH1, but not TTYH2 and 3, was initially described as putative maxi-chloride anion channels, activated by hypo-osmotic solution (HOS) [39], which was disproven later [40]. Recently, TTYH1 was confirmed to be a potent regulator of tumor microtubule morphology, invasion, and proliferation of glioma, which are the known functions of VRACswell [41]. Brain transcriptome analysis by RNA sequencing showed that in astrocytes TTYH1 and 3 were the top 49th and 94th among 22,458 genes ranked by expression level [42]. Furthermore, Ttyh1/2/3 showed 11-, 12-, and 3-fold higher mRNA level in astrocyte compared to neuron in the brain [42]. Therefore, Ttyh1/2/3 should be considered as potential candidates of the astrocytic VRACswell.
In this study, we set out to determine the true molecular identity of the astrocytic VRACswell, activated by AQP4-dependent swelling. Many previous studies of VRACΓ and VRACswell have used external and internal solutions that contained cations such as Na+, K+, and Cs+, which might have allowed the investigators to inadvertently record currents contaminated by cations. We have utilized a special recipe of external and internal solutions consisting only Cl− as the permeating ion to eliminate any contribution of cations Na+, K+ and to minimize Cs+ for the recording of genuine ICl,swell, as previously described for the recording of VRACswell current in cultured astrocyte [6]. We have identified and characterized Ttyh1/2/3, not LRRC8A nor BEST1, as the pore-forming subunits of the astrocytic VRACswell.
MATERIALS AND METHODS
C57BL/6J mice and hGFAP-CreERT2 were used at 8~10 weeks of age. The hGFAP-CreERT2 mice are inducible transgenic mice under control of human
Primary cultured astrocytes were prepared from cortex of C57BL/6 mouse pups (P0-P2) as described [37]. The cerebral cortex was dissected from the brain and adherent meninges were removed, minced and dissociated into single cell suspension by trituration. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, #10-013, Corning) supplemented with (in mM) 25 glucose, 4 L-glutamine, 1 sodium pyruvate, 10% heat-inactivated horse serum (#260500-088, Gibco), 10% heat-inactivated fetal bovine serum (#10082-147, Gibco) and 10,000 units/ml penicillin-streptomycin (#15140-122, Gibco). Cultures were maintained at 37℃ in a humidified 5% CO2 incubator. On DIV3, cells were vigorously washed with repeated pipetting and the media was replaced to get rid of debris and other floating cell types. During maintaining the culture before use, the media was replaced every 3~4 days. For gene-silencing experiments with primary cultured astrocytes, various shRNA vectors were electroporated (Neon Transfection system kit; #MPK10096, Invitrogen) into trypsinized cultured astrocytes 4 days before the experimental day and replated onto culture dish. One day before the experimental day (for recording ICl,swell, DIV21 primary astrocyte culture were used), cells were replated onto cover-glass coated with 0.1 mg/ml Poly D-Lysine (PDL, #P6407, Sigma-Aldrich).
Human embryonic kidney 293T (HEK293T) and Chinese hamster ovary-K1 (CHO-K1, referred as CHO) cells were purchased from ATCC (#CRL-3216, ATCC) and the Korean Cell Line Bank (Seoul National University, Republic of Korea), respectively. All cell lines have been tested for mycoplasma contamination. HEK293T cells and CHO cells were cultured in DMEM (#10-013, Corning) and F-12 (#21127-022, Gibco), respectively. Both media were supplemented with (in mM) 25 glucose, 4 L-glutamine, 1 sodium pyruvate, 10% heat-inactivated fetal bovine serum (#10082-147, Gibco) and 10,000 units/ml penicillin-streptomycin (#15140-122, Gibco). Cell lines were maintained at 37℃ in a humidified atmosphere of 95% air and 5% CO2. 18 hours before the experimental day, cells were transfected with DNA clone by transfection reagent (Effectene, #301425, Qiagen). On experiment day, the transfected cells were replated onto cover-glass for electrophysiological recordings. For immunocytochemistry, HEK293T cells were plated onto PDL coated cover-glass, and transfected with DNA clone 20 hours before fixation.
Various kinds of shRNA were synthesized as for following: Two kinds of oligos were purchased (Sequence of oligos; [phos]5′-t [sense sequence of target] ttcaagaga [reverse complement sequence of target] ttttttc-3′ and [phos]5′-tcgagaaaaaa [sense sequence of target] tctcttgaa [reverse complement sequence of target] a-3′). The sequence information for control shRNA and various candidates of shRNA are listed in Table 1.Two oligos were annealed with annealing buffer (in mM; 200 potassium acetate, 60 HEPES-KOH, 4 Mg-acetate, pH 7.3 was adjusted by KOH) and incubated at 95℃ for 5min and 70℃ for 10 min. The annealed double-stranded oligo was inserted into HpaI-XhoI restriction enzyme sites of pSicoR lentiviral vector [44] (#11579, Addgene) and verified by sequencing. The shRNA containing pSicoR vectors were electroporated into cultured astrocytes. In experiments using triple combination of
The 7~9 weeks old male mice were anesthetized with 2% tribromoethanol (Avertin, 20 µl/g) and placed in a stereotaxic frame. The scalp was incised and exposed the skull. The connective tissue was gently scraped away. Stereotaxic coordinates were: AP: −1.7 mm; L: 1.7 mm; and DVL:−1.85 mm, according to Allen Mouse Brain Atlas (http://www.brain-map.org). The cocktail of lentiviruses carrying either (pSicoR-
The tamoxifen-inducible Cre-mediated recombination is expected to result in deletion of the floxed sequences. To activate Cre recombinase, 7~9 weeks-old male mice were administrated with tamoxifen (#T5648, Sigma-Aldrich) once a day (200 mg per kg of body weight, intraperitoneal) for 7 days. Tamoxifen was dissolved in sunflower oil (#1642347, Sigma-Aldrich) with 10% ethanol at a final concentration of 20 mg per milliliter at room temperature, and stored at 4℃ in the dark. The shRNA is floxed with loxP sites in the pSicoR construct, which is cleaved by Cre-expression [44]. The cocktail of lentiviruses carrying pSicoR-
The design for
All truncation mutant and single amino acid mutants of TTYH1 were constructed in shRNA-insensitive TTYH1 tagged with GFAP clone (
4-(2-butyl-6,7-dichloro-2-cyclopentylindan-1-on-5-yl)oxybutyric acid (DCPIB), 2-(2-Amino-3-methoxyphenyl)-4
For VRAC current (herein defined as Cl− current induced by hypo-osmotic stimulation, ICl,swell) recording, primary cultured cortical astrocyte or transfected HEK292T or CHO cells, and acute hippocampal slices from 8~10 weeks mice were used for
Standard external bath solutions of both ISO and HOS for VRACswell were composed of (in mM): 70 Tris-HCl, 1.5 CaCl2, 10 HEPES, and 10 glucose, 5 TEA-Cl, 5 BaCl2 and adjusted to pH 7.3 with CsOH, as previously described [6]. The osmolality of each solution was adjusted with sucrose: 100 mM sucrose for 280~290 mOsm (for ISO) and 30 mM sucrose for 220~230 mOsm (for HOS), Solution osmolality was confirmed by a vapor pressure osmometer (Vapro osmometer #5600, Wescor). The external solution of both ISO(NaCl) and HOS(NaCl) for recording of LRRC8-mediated VRACΓ was composed of (in mM) : 150 NaCl for ISO(NaCl), 105 NaCl for HOS(NaCl), respectively, 6 KCl, 1 MgCl2, 1.5 CaCl2, 10 Glucose, 10 HEPES, adjusted to pH 7.4 with NaOH. The solution osmolality is 320mOm for ISO(NaCl) and 240mOsm for HOS(NaCl). For Ca2+ dependency with TTYH mediated ICl,swell, 1.5 mM CaCl2 is changed to 0.15 mM CaCl2 for all external bath solution. TEA-Cl and BaCl2 were added to eliminate the potassium currents enabling selective recording of Cl− current. For glutamate permeability experiments, 70 mM Tris-Cl containing HOS external solution was substituted for 70 mM Tris-base and 70 mM glutamate (glutamate containing HOS, gHOS) to omit external Cl−. When the maximal current was generated after standard HOS treatment, the standard HOS (70 mM Tris-Cl) was changed to gHOS (70 mM Tris-glutamate). The reversal potentials from both standard HOS (70mM Tris-Cl) and gHOS (70mM Tris-glutamate) were measured to calculate the ratio of glutamate permeability (detailed description in the analysis of electrophysiological data).
For the recording of various anion (SCN−, I−, Br−, and F−) permeability, 105mM of NaCl in HOS (NaCl) was changed to the same concentration, 105mM of NaSCN, NaI, NaBr, and NaF at the maximal conductance of VRACswell or VRACΓ recording. The reversal potentials from each HOS(NaCl) (105mM NaCl) and HOS(NaX−) (105mM NaSCN, NaI, NaBr, and NaF) were measured to calculate ratio of ion permeability (The calculating equation for of relative anion permeability is the same with glutamate permeability, detailed description in analysis of electrophysiological data).
Standard internal solution for VRACswell was composed of (in mM) : 60 Trizma-HCl, 70 Trizma-base, 70 Aspartic acid, 15 HEPES, 0.4 CaCl2, 1 MgCl2, 4 Mg-ATP, 0.5 Na-GTP, and 1 EGTA, adjusted to pH 7.25 with CsOH, as previously described [6].
The internal solution for recording of LRRC8-mediated VRACΓ was composed of (in mM) : 40 CsCl, 100 CsMeS, 1 MgCl2, 1.9 CaCl2, 5 EGTA, 4 Na2ATP, 10 HEPES, adjusted to pH 7.2 with CsOH, as previously described(Voss et al., 2014). For Ca2+ free experiment, 1mM EGTA was replaced into 20mM BAPTA which leads 100nM free Ca2+ to be reduced by 10nM. Patch pipettes had tip resistances of 5~8MΩ when filled with internal solution. Pipettes were pulled from borosilicate thin-wall glass capillaries (TW150F-4, World Precision Instruments) with the micropipette puller (P-97, Sutter Instrument). Unlike other previous studies [25,26], we used Tris·Cl-based external solution without Na+ and K+ and minimal level of Cs+ (15mM of CsOH for pH adjustment of external solution), and Tris·Cl-based internal solution to eliminate any contribution of Na+, K+ and to minimize Cs+ to ICl,swell. This is particularly important for isolating the anionic current (See Table 2).
For substituted-cysteine accessibility method (SCAM), 10 amino acids in TTYH1 (from V161 to A170) were each substituted with cysteine residue. The cysteine mutant of TTYH1 or TTYH1-WT were co-transfected with AQP4 in HEK29T cells. The standard bath solution of HOS containing MTSES (100mM) was applied at the maximal ICl,swell. The percent block was determined.
To test the dominant negative function of TTYH1-R165A, pEGFP-N1-TTYH1-WT and pEGFP-N1-TTYH1-R165A were co-expressed with mAQP4-M1-mcherry in HEK293Tcells. The total DNA amount of TTYH1 (WT or R165A) is 2 mg and AQP4 is 750ng. The relative amount of R165A to WT is varied with 1:0, 0.75:0.25, 0.5:0.5, 0.25:0.75, and 0:1. The maximal current of ICl,swell was recorded in each condition by treatment of a standard solution of HOS.
To identify shRNA-transfected or infected astrocytes from primary cultures or hippocampal slices, GFP or mcherry fluorescence was used (Fig. 10D and 11J), which were driven under CMV promoter in pSicoR vector construct (Fig. 11I). In the case of hippocampal slices, astrocytes were distinguished by electrophysiological properties such as low membrane resistance (Rm), low resting membrane potential after rupturing. The whole cell configuration was achieved by rupturing the cell membrane with suction after achieving a giga-seal. Holding voltage was −60 mV. Stability and quality of the patch were determined by monitoring the cell parameters: Rm, cell capacitance (Cm), and series resistance (Ra).
The episodic ramp protocol (+100 mV to −100 mV, 1000 ms, 15 s interval) for measurement of the maximal conductance of VRACswell and VRACΓ and the current step protocol (from−100 mV to +140 mV with 20 mV step) for measurement of the voltage-dependent inactivation of VRACswell and VRACΓ were given and recorded with a Digidata 1322A interface and pClamp10 software (Molecular Devices). A continuous gap-free recording was simultaneously conducted with Minidigi digitizer and Axoscope10 software (Axon Instruments).
For drawing the I~V curve for ICl,swell, the averaged mean value and standard error of mean (SEM) at every 5 mV are plotted. The current density (pA/pF) of ICl,swell was measured by subtraction of maximal Cl− current (average of maximal 4weeps in HOS treatment) from basal Cl− current (average 4weeps of ISO treatment, 1 min before HOS treatment) divided by the capacitance of the membrane (Cm), and measured the amplitude of current density (pA/pF, from −100 mV to 100 mV) by ICl,swell (at 100 mV) – ICl,swell (at −100 mV). Rectification index was defined here as the following equation.
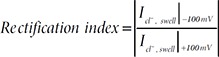
To examine the glutamate permeability (PGlu) of VRACs, we measured the reversal potential shift of ICl,swell caused by external solution change from standard HOS solution (70 mM Tris-Cl) to gHOS (70 mM Tris-glutamate) solution, which was corrected for junction potential (9.4mV). The detailed composition of the gHOS solution is described above. The reversal potential of ICl,swell was calculated with following Goldman-Hodgkin-Katz (GHK) equation.
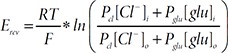
With this equation, reversal potential difference (Δ
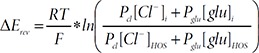
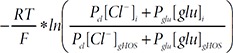
The ‘i’ and ‘o’ indicates internal and external solution respectively. HOS and gHOS represent standard hypo-osmotic stimulation condition and glutamate containing HOS condition respectively.
For IOS imaging devices, Infrared (IR) light source with optical filter (775 nm wavelength, Omega Filters) was used for transillumination of brain slices and these optical signals were obtained as IOS images using a microscope (BX50WI, Olympus) equipped with a CCD camera (ORCA-R2, Hamamatsu). Imaging Workbench software (INDEC Biosystems) was used for image acquisition and image analysis.
For the detailed procedure of experiments, we first prepared mouse brain hippocampal slices (described in the methods for electrophysiology). Next, we fixed hippocampal slice into the recording chamber and positioned electrical stimulator in the CA1 stratum radiatum region. We determined the region of interest (ROI) by calculating 1.5~2% increase in IOS by single pulse electrical stimulation (20Hz, 1 sec, 200~300mA). Images were acquired every 1 second and after stabilization of the IOS signal, we acquired 10 min baseline IOS transmittance (Tbase). Next, we applied 1Hz-30min electrical stimulation (which has been previously shown to induce activity-dependent cell swelling [2]) and acquired two IOS indexes required for analysis: maximum IOS transmittance (Tmax) and final IOS transmittance (Tend). With these defined indicators, we calculated the induced maximal volume increase (b) and regulated volume decrease (a) as Tmax−Tbase and Tmax−Tend respectively (Fig. 11C, E, and J). Finally, the percentage of regulated volume decrease (RVD%) was calculated by

Gene silencing test with shRNAs that target VRAC candidates including
The knock-down efficiency of
To determine whether the direction of N-, C-terminus, and Loop1 of TTYH1 are located in the cytosolic or extracellular side, HEK293T cells were transfected with TTYH1-EGFP or TTYH1-GFP (Loop1-FLAG), grown on coverslips for 24 hours. The cells were fixed in 4% paraformaldehyde for 30 min at room temperature. The permeabilized group are treated PBS with 3% Triton X-100 for 5 min and the non-permeabilized group is treated PBS for 5min. Non-specific binding was prevented with 1-hour incubation in 2% donkey serums. Cells were incubated the rabbit anti-Ttyh1 (for N-terminus of TTYH1, #NBP1-59909, Novus Biologicals), anti-GFP (for C-terminus of TTYH1, #ab13970, Abcam), and anti-FLAG (for Loop1 of TTTYH1, #F1804, Sigma-Aldrich) primary antibodies for overnight at 4℃. After washing, DyLight 594-conjugated secondary antibody (Jackson lab, 1:400) was added and incubated for 2 hours at room temperature. The cells were washed and mounted, and then observed under a Nikon A1 confocal microscope.
Mice under anesthesia with 2% tribromoethanol (avertine, 20 µl/g) and were trans-cardially perfused with 0.9% saline followed by 4% PFA, and then the brains were removed. The dissected brains were post-fixed with 4% PFA at 4℃ for 18~24 hours and dehydrated with 30% sucrose at 4℃ for 48 hours. For immunostaining, brain sections with a thickness of 30 µm were cut using a cryostat, blocked with the serum (donkey serum 2%, goat serum 2%, the solution including 0.3% Triton X-100) of the appropriate species for 1 hour and then treated with primary antibodies, including rabbit anti-TTYH1 (1:200, #NBP1-59909, Novus Biologicals), chicken anti-GFAP (1:500, #ab5541, Millipore), mouse anit-NeuN (1:500, #MAB377, Millipore), DAPI (1:5000, #46190, Chemicon). During over-night. The next day, tissues were rinsed 3 times with 0.1M PBS and subsequently incubated with secondary antibodies conjugated with Alexa Fluor 488, 594 and 647 for 1 hour 30 min. After treating with secondary antibodies, the tissues were washed out with PBS including DAPI staining in the second washing step. After three rinses in PBS, the sections were mounted on slide glasses. Images were acquired on a Nikon A1 confocal microscope.
Statistical analyses were performed using ImageJ software (NIH Image). For analyzing the knock-down efficiency of AAV-virus containing control or
Publically available brain RNA-seq data sets [42] of VRAC candidates such as TTYH, LRRC8, BEST family were used in Fig. 1D (https://web.stanford.edu/group/barres_lab/brain_rnaseq.html). Multiple alignments of amino acids were processed from CLUSTAL OMEGA (http://www.ebi.ac.uk/Tools/msa/clustalo/). The dendrogram was drawn by the PHYLIP program. The prediction of the transmembrane domain of TTYH family was processed by TMHMM (http://www.cbs.dtu.dk/services/TMHMM/). The potential phosphorylation site and kinases were predicted by GPS 3.0 (http://gps.biocuckoo.org/online.php). Statistical analysis was performed using GraphPad Prism 7.01 software. p<0.05 was considered statistically significant. No statistical methods were used to predetermine sample size. All statistical analysis results and methods were described in the Supplemental Table 1 for main figures.
Statistical parameters including the exact value of n, the definition of center, dispersion and precision measures (mean ± SEM) and statistical significance are reported in the Supplemental Table 1. All data points are tested if the values come from a Gaussian distribution by D'Agostino-Pearson omnibus normality test, and then appropriate statistical methods are applied. In figures, asterisks denote statistical significance as *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.000, as well as non-significance with NS, p>0.05. Statistical analysis was performed in GraphPad PRISM 7.
RESULTS
Previous studies suggested that water movement via AQP4 is critical for an initial volume increase which then somehow triggers an opening of VRACswell in astrocytes [46]. To test the requirement of AQP4 for activation of VRACswell, we recorded ICl,swell under voltage-clamp configuration from cultured mouse astrocytes expressing either control or
Previously, two groups concurrently reported that LRRC8A is an essential component of VRACΓ in various cell lines [25,26,27]. Thus, we tested whether LRRC8A is the major component of VRACswell in astrocytes. We developed a specific shRNA for
It has been subsequently contended that BEST1, not LRRC8A, is indispensable for volume regulation in hiPSC-RPE cells [36]. Over the years, we have extensively characterized BEST1 as a Ca2+-activated anion channel, localized at peri-synaptic sites of the hippocampal astrocytes, functioning as the glutamate- and GABA-permeable channel in cerebellum and hippocampus, respectively [47]. To test whether there is any contribution of BEST1 in the astrocytic VRACswell, we utilized previously characterized BEST1 knockout mouse [48] and
We next focused on
The reduction of ICl,swell by
To test if each TTYH isoform confers VRACswell activity, we transiently expressed each TTYH isoform clone in HEK293T cells together with AQP4 to maximize HOS-induced volume increase and recorded ICl,swell (Fig. 3A~C). We found that each TTYH isoform co-expressed with AQP4 showed significantly increased ICl,swell compared to the condition of AQP4 alone (Fig. 3A~C), indicating that all three isoforms of TTYH are sufficient for activation of the ICl,swell in the presence of AQP4 and that each isoform of TTYH family can mediate VRACswell possibly as a homomeric channel. In marked contrast, LRRC8A has been repeatedly shown to be insufficient for VRACswell activity in heterologous expression systems [25,26,28,31]. Consistently, we did not observe ICl,swell in CHO-K1 cells co-expressing LRRC8A and AQP4 (Fig. 3D~F), indicating that LRRC8A is not sufficient for VRACswell.
The unexpected lack of HOS-induced ICl,swell in naïve HEK293T cells (Fig. 3A~C) was a surprising observation which called for further investigation. In the past, LRRC8A-mediated VRACΓ in naïve HEK293T cells has been recorded with external solution containing cations such as Na+, Cs+, or K+ (see Table 2 in Materials and Methods). In contrast, the external solutions that we have utilized contain minimal cations as previously described for the recording of astrocytic VRAC [6]. Therefore, we directly tested the two different solutions in native HEK293T cells. By surprise, we found that ICl,swell was not observed when the native HEK293T cells with endogenous LRRC8A-mediated VRACΓ was treated with the HOS which contained minimal cations, whereas a robust ICl,swell was induced by the Na+-containing HOS (Fig. 3G~I), suggesting that an activation of LRRC8A either requires external cation or possesses a sensitivity to Tris. To further confirm whether this ICl,swell induced by Na+-containing HOS was mediated by LRRC8A, we engineered previously reported
The astrocytic ICl,swell was reported to display an outwardly rectifying conductance [6] with a calculated rectification index to be less than one. The rectification index of each Ttyh1/2/3-mediated ICl,swell was also identical to that of ICl,swell recorded from cultured astrocytes with a strong outward rectification (Fig. 4E). Crepel et al. also showed that the Ca2+ is not essential for the activation of astrocytic VRACswell [6]. We observed that each of the Ttyh1/2/3-mediated ICl,swell is also independent of Ca2+ as evidenced by the similar amplitude of ICl,swell in low external Ca2+ (0.15mM) and high internal Ca2+-chelator BAPTA (20mM) (Fig. 4F~H). We next examined the pharmacological profiles of astrocytic VRACswell compared to Ttyh1/2/3-mediated ICl,swell in HEK293T cells. DCPIB is a known inhibitor for both VRACswell and VRACΓ recorded from various cell lines including cultured astrocytes [50,51]. We found that DCPIB rapidly blocked ICl,swell in cultured astrocytes (Fig. 4I~K) and Ttyh1/2/3-mediated ICl,swell in HEK293T cells (Fig. 4L and 4M). Taken together, these results indicate that Ttyh1/2/3 share the same biophysical (outward rectification), biochemical (Ca2+-independency) and pharmacological (sensitivity to DCPIB) properties as the astrocytic VRACswell.
As previously reported, tyrosine kinase (TK) and mitogen-activated protein kinase (MAPK) are essential for activation of the astrocytic VRACswell [6]. We also confirmed that pre-treatment of TK inhibitor genistein (Fig. 5A~C) and MAPK inhibitor PD98059 (Fig. 5E~G) completely abolished ICl,swell in cultured astrocytes. Each of the Ttyh1/2/3-mediated ICl,swell was similarly blocked by pre-treated genistein (Fig. 5A and 5D) or PD98059 (Fig. 5H~J). In fact, the surface expression of TTYH1 was enhanced by HOS treatment, which was prevented by either TK or MAPK inhibitor (Fig. 5K). These results indicate that each TTYH isoform shares the identical kinase-dependency as the astrocytic VRACswell. In contrast, LRRC8A-mediated VRACΓ showed a profoundly different sensitivity to MAPK inhibitor (Fig. 5L~N).
Another unique property of the astrocytic VRACswell is its relatively high PGlu/PCl [8,32,52]. To measure the PGlu/PCl of ICl,swell, we exchanged the external solution from HOS (83 mM Cl−) to glutamate-containing HOS (gHOS: 70 mM glutamate and 13 mM Cl−) after reaching a maximal current amplitude of ICl,swell (Fig. 5O). We found that in both cultured astrocyte (Fig. 5P and 5Q) and each Ttyh1/2/3-expressing HEK293T cells (Fig. 5R), the reversal potential of ICl,swell shifted to a positive potential in gHOS treatment. Based on the reversal potential shift, we calculated the PGlu/PCl according to Goldman-Hodgkin-Katz equation and found that ICl,swell in cultured astrocytes and each Ttyh1/2/3-mediated ICl,swell shared the similar high PGlu/PCl at around 0.26 (Fig. 5S). Taken together, these results indicate that kinase-dependency and glutamate-permeability of each TTYH-mediated ICl,swell resemble those of the astrocytic VRACswell.
If TTYH isoforms are the true channel-forming subunit of VRACswell, we should be able to determine the channel-pore as a single amino acid residue. To identify the exact pore-forming amino acid residue, we first utilized bioinformatical approaches to predict the membrane topology of TTYH isoforms. Based on the dendrogram analysis (Fig. 6A) and sequence alignment (Fig. 7) of Ttyh1/2/3 isoforms across different species, we found that TTYH1, which is the most abundantly expressed isoform in astrocytes (Fig. 1D), showed the order of sequence homology: TTYH1>TTYH2>TTYH3 >>
Because channel-pores usually reside within a loop between two transmembrane domains, we next closely examined the loop-sequences of Loop1-4 of TTYH1 (Fig. 8C) and found that Loop2 and Loop4 were highly conserved across different species but not Loop1 (Fig. 7). Loop3 appeared to be too short for a pore. Therefore, we focused on Loop2 and Loop4 to search for the channel-pore. We generated a series of truncation mutants (Δ) with a 20 amino-acid-deletion for each loop2 and 4 and also recorded ICl,swell. We identified potential candidates, Δ2A (S116-T135) and Δ2C (T156-A175) in Loop2 and Δ4B (L289-N304) in Loop4, which all showed an elimination of ICl,swell (Fig. 8D). Then, we performed surface biotinylation assay for each candidate to eliminate the possibility that the disappearance of ICl,swell is due to an impaired surface expression of the channel protein. We eliminated Δ4B in Loop4 and ΔYY (ΔY299, Y300) in Loop4B as a potential pore because of the lack of surface expression of Δ4B and ΔYY (Fig. 8E). We made additional truncation mutants with Δ2A and Δ2C into 5-amino-acid-deletions and narrowed down to Δ2A4, Δ2C2, and Δ2C3, which showed significantly reduced ICl,swell (Fig. 8D). After examining the surface biotinylation results (Fig. 8E), we found that the positively charged arginine residue at 165 position (R165) satisfied the definition of putative single amino acid pore residue, displaying a signficant reduction of ICl,swell (Fig. 8F and 8G) and a positive shift in the reversal potential of about +7 mV (Fig. 8G) without affecting the surface expression after substitution with alanine (R165A) (Fig. 8E). This result suggests that the TTYH1-R165A is less permeable to Cl− compared to TTYH1-WT. The substitution at the homologous residue, R164A in TTYH2 similarly showed significantly reduced ICl,swell (Fig. 8H and 8I) indicating that the arginine residue (R165 for TTYH1 and R164 for TTYH2) is the key pore-forming residue. We further found that R165A mutant of shRNA-insensitive TTYH1, when co-expressed with all
To determine whether R165 and neighboring residues in TTYH1 are located at the pore lining vestibule, we performed the SCAM with cysteine substituted mutants of each of 10 amino acids from V161 to A170 (Fig. 9A~D). The control TTYH1-WT mediated ICl,swell was reduced by 9.01% with 100mM of MTSES which has no permeability through the plasma membrane, suggesting that there is a basal level sensitivity of TTYH1 for 100mM of MTSES (Fig. 9A~C). Among 10 amino acids, R165C was most significantly blocked by 100mM of MTSES with the highest block percentage (36.58%) (Fig. 9A~C), suggesting that R165 is the
Due to the lack of molecular identity, the brain distribution and
The primary role of VRACswell in volume regulation is its participation during RVD. To recapitulate RVD in hippocampal slices, we performed intrinsic optical signal (IOS) imaging which represents the astrocytic volume change through water movement via AQP4 upon repetitive neuronal activity [2,55]. Among various stimulation protocols, we found that low-frequency stimulation (LFS; 1 Hz) for 30 min at Schaffer-collateral pathway caused an RVD with a continuous increase in IOS, which peaked at around 15 min, followed by a steady decrease in IOS until the end of 30 min stimulation period (Fig. 11A~C, and 11E~G). In contrast, a brief 20 Hz or 100 Hz stimulation only casued a transient IOS, but not DCPIBsensitive RVD (Fig. 11D). The LFS-induced RVD was completely blocked by DCPIB and genistein treatments (Fig. 11E~G), raising a possibility that the LFS-induced RVD might be mediated by Ttyh1/2/3.
To test whether LFS-induced RVD is mediated by TTYH isoforms, we injected lentivirus carrying all
DISCUSSION
Our study elucidates for the first time that TTYH family of genes encode the pore-forming subunits of VRACswell in the brain. Several lines of evidence support this conclusion: (1) The gene-silencing experiments using all three
One of the unique properties of an ion channel family is its ability to assemble as a homomeric or heteromeric complex to form a functional channel. For TTYH family there are three possible scenarios: (1) each TTYH subunits can assemble as a homomer; (2) TTYH subunits can assemble as a heteromer within TTYH family; and (3) TTYH subunits can assemble as a heteromer with other unknown proteins. Although we cannot completely rule out the possibilities of TTYH subunits assembling as heteromers, our results support the possibility that TTYH subunits can assemble and function as a homomer. If TTYH subunits were assembled only as a heteromer within TTYH family, gene-silencing by the combination of two shRNAs should have been effective to reduce the ICl,swell in astrocytes. However, this was not the case: Only the combination of all three shRNAs (Fig. 2E, 2G and 2H), but not the combinations of two shRNAs (Fig. 2E and 2F), was able to significantly and fully silence the ICl,swell in astrocytes. These strongly suggest that TTYH subunits can assemble and function at least as a homomer. It is also unlikely that TTYH subunits assemble with other unknown protein to form a functional channel because heterologous overexpression of each TTYH subunits produced an equal magnitude of ICl,swell in HEK293T and CHO-K1 cells, which are originated from human kidney and hamster ovary, respectively. The chance of such unknown protein to be expressed at high level in those two seemingly different cell lines and astrocytes is very low. More importantly, the strongest evidence for homomeric assembly of TTYH in astrocytes comes from the observation that co-expression of shRNA-insensitive TTYH1-WT with all three Ttyh1/2/3 shRNAs fully rescued ICl,swell (Fig. 2E, 2G and 2H), whereas co-expression of the pore-mutant TTYH1-R165A (shRNA-insensitive) and all three Ttyh1/2/3 shRNAs caused a complete impairment of ICl,swell (Fig. 8J and 8K). Therefore, we propose that astrocytic VRACswell is composed of mostly homomers of all three Ttyh1/2/3 subunits. Nevertheless, further studies are needed to clarify the exact subunit composition of the TTYH channel.
In this study, we provide unprecedented evidence that each Ttyh1/2/3 isoform acts as a pore-forming subunit of VRACswell, while LRRC8A acts as a pore-forming subunit of VRACΓ, which modulates the speed of cell swelling and subsequent activation kinetics of VRACswell. These ideas are derived from the detailed analysis of activation kinetics of ICl,swell recorded from both
In support of the possibility of an indirect interaction between TTYH-mediated VRACswell and LRRC8-mediated VRACΓ, a recent report provided a crystal structure of the pore-forming subunits of LRRC8 family [29]. In this report, the authors clearly demonstrated that activation of LRRC8 requires reduced cytosolic ionic strength, but not swelling or membrane stretch [27,29]. Based on this report and our findings, we now propose a mechanistic model (Fig. 12A) to give a more comprehensive view and to explain the observed difference in activation kinetics of ICl,swell after
The precise molecular mechanisms of sensing the cell swelling induced by hypo-osmotic shock, leading to an opening of VRACswell are poorly understood. Several hypothetical models for sensing the cell-swelling have been proposed [57,58,59]; (1) direct sensing of membrane stretch, (2) direct or indirect tethering to the cytoskeleton, (3) alterations in membrane curvature such as caveolae complex by mechanical or membrane-compositional changes, and (4) via interaction with integrins activated by the cell volume perturbation, and (5) via swelling-induced interaction between actin-binding protein (ACTN4) and a cytosolic member of ABC transporter superfamily (ABCF2) prevents ABCF2 from suppressing VRACswell. Previous studies supporting these models have identified caveolin-1 and caveolin-related proteins that form a membrane curvature called caveolae in astrocytes [60]. This caveolin-1 is tethered to actin microfilaments [61] and positively modulates the activity of VRAC via c-Src tyrosine kinase [62]. Meanwhile, integrins are known to be associated with not only the extracellular matrix but also caveolin and positively regulate VRAC activation via various kinases such as FAK tyrosine kinase [63]. It has been previously demonstrated that a5-integrin is co-localized with TTYH1 in TTYH1-GFP over-expressing HEK293T cells [64]. We have demonstrated that Ttyh1/2/3-mediated ICl,swell is fully blocked by genistein and PD98059, which are the inhibitors of tyrosine kinase and MAP kinase, respectively (Fig. 5A, 5D and Fig. 5H~J). Based on these results, we can propose a mechanistic insight that there exists a complex of caveolin, integrin, cytoskeleton, and TTYH channels at astrocytic membrane curvature called caveolae, and hypo-osmotic shock somehow transduces through the integrin-caveolin-cytoskeleton complex to activate MAP kinase and tyrosine kinase to phosphorylate TTYH channels, which is required for either activation or surface expression of TTYH channels (Fig. 12B). In support of this possibility, we have observed that the surface expression of TTYH1 is enhanced by HOS treatment and prevented by either TK or MAPK inhibitor (Fig. 5K). These exciting possibilities await future investigations.
In addition to the fundamental role of VRACswell in cellular volume regulation, VRACswell is also implicated in many other functions including regulation of cell proliferation, apoptosis and motility, which occur during an early developmental stage in the embryonic neural stem cells [17,18]. Consistently, TTYH1 has been shown to be prominently expressed in embryonic stem cells during developmental stages and various brain structures in developing and adult brain, while limited expression was detected in non-neural tissues and cell types [65]. In the brain, TTYH1 appears to be associated with the general functions of VRACswell such as proliferation, apoptosis, and motility. For example, TTYH1 expression was shown to be increased at the edge of extending processes of the migrating cultured astrocytes after a scratch injury [66], suggesting its role in migration. TTYH1 expression is also increased in hypertrophied reactive astrocytes following
We have demonstrated that TTYH channels are highly permeable to glutamate (PGlu/PCl = 0.2~0.3) (Fig. 5O~S), as VRACswell has been already shown to release glutamate and other osmolytes [8,9,10,15,51]. We have also demonstrated that 1 Hz stimulation in the Schaffer-collateral pathway in the hippocampus causes a profound volume increase and eventual TTYH1-mediated RVD (Fig. 11J~L). It is possible that glutamate released through TTYH channels during RVD might regulate synaptic transmission as well as synaptic plasticity. Consistent with this idea, it has been previously shown that taurine, released through DCPIB-sensitive VRACswell, can mediate glycinergic neurotransmission in the hypothalamus [11]. We show that there is no contribution of DCPIB-sensitive RVD after a brief 20 Hz or 100 Hz stimulation at Schaffer-collateral pathway in the hippocampus (Fig. 11D). In contrast, a prolonged 1 Hz stimulation, which is a conventional protocol for long-term depression (LTD) at this synapse, causes a profound increase in volume and initiation of RVD (Fig. 11C, Fig. 11E~G, J~L). During such RVD, glutamate and other osmolytes are expected to be released through TTYH channels and affect synaptic transmission as well as NMDA receptor- or mGluR-dependent synaptic plasticity, in particular, LTD. These exciting possibilities should be explored in the future.
In conclusion, with the advent of molecular identification of TTYH family as the VRACswell of the brain, the putative functions of VRACswell can be systematically addressed at the molecular, cellular and systemic levels in the future. Furthermore, our study should provide novel therapeutic targets for cerebral edema following ischemic stroke, brain trauma, and brain cancer, which are known to be associated with VRACswell [14,67].
SUPPLEMENTARY MATERIALS
Figures












Tables
References
- Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci 2013;14:265-277.
- Woo J, Kim JE, Im JJ, Lee J, Jeong HS, Park S, Jung SY, An H, Yoon S, Lim SM, Lee S, Ma J, Shin EY, Han YE, Kim B, Lee EH, Feng L, Chun H, Yoon BE, Kang I, Dager SR, Lyoo IK, Lee CJ. Astrocytic water channel aquaporin-4 modulates brain plasticity in both mice and humans: a potential gliogenetic mechanism underlying language-associated learning. Mol Psychiatry 2018;23:1021-1030.
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 1997;68:69-119.
- Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia 2004;46:419-436.
- Hazama A, Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J Physiol 1988;402:687-702.
- Crépel V, Panenka W, Kelly ME, MacVicar BA. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J Neurosci 1998;18:1196-1206.
- Kimelberg HK, Macvicar BA, Sontheimer H. Anion channels in astrocytes: biophysics, pharmacology, and function. Glia 2006;54:747-757.
- Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y, Li Y, Yang J, Dienel G, Zielke HR, Nedergaard M. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci U S A 2005;102:16466-16471.
- Liu HT, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia 2006;54:343-357.
- Liu HT, Akita T, Shimizu T, Sabirov RZ, Okada Y. Bradykinin-induced astrocyte-neuron signalling: glutamate release is mediated by ROS-activated volume-sensitive outwardly rectifying anion channels. J Physiol 2009;587:2197-2209.
- Choe KY, Olson JE, Bourque CW. Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus. J Neurosci 2012;32:12518-12527.
- Tucker B, Olson JE. Glutamate receptor-mediated taurine release from the hippocampus during oxidative stress. J Biomed Sci 2010;17:S10.
- Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg 1995;83:1051-1059.
- Kimelberg HK. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia 2005;50:389-397.
- Zhang Y, Zhang H, Feustel PJ, Kimelberg HK. DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra. Exp Neurol 2008;210:514-520.
- Ransom CB, O'Neal JT, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci 2001;21:7674-7683.
- Okada Y, Sato K, Numata T. Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 2009;587:2141-2149.
- Hoffmann EK, Holm NB, Lambert IH. Functions of volume-sensitive and calcium-activated chloride channels. IUBMB Life 2014;66:257-267.
- Shimizu T, Lee EL, Ise T, Okada Y. Volume-sensitive Cl− channel as a regulator of acquired cisplatin resistance. Anticancer Res 2008;28:75-83.
- Lee EL, Shimizu T, Ise T, Numata T, Kohno K, Okada Y. Impaired activity of volume-sensitive Cl− channel is involved in cisplatin resistance of cancer cells. J Cell Physiol 2007;211:513-521.
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol 1997;273:C755-C789.
- Clapham DE. The list of potential volume-sensitive chloride currents continues to swell (and shrink). J Gen Physiol 1998;111:623-624.
- Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand 2003;177:119-147.
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 2009;89:193-277.
- Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 2014;157:447-458.
- Voss FK, Ullrich F, Münch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 2014;344:634-638.
- Syeda R, Qiu Z, Dubin AE, Murthy SE, Florendo MN, Mason DE, Mathur J, Cahalan SM, Peters EC, Montal M, Patapoutian A. LRRC8 proteins form volume-regulated anion channels that sense ionic strength. Cell 2016;164:499-511.
- Okada T, Islam MR, Tsiferova NA, Okada Y, Sabirov RZ. Specific and essential but not sufficient roles of LRRC8A in the activity of volume-sensitive outwardly rectifying anion channel (VSOR). Channels (Austin) 2017;11:109-120.
- Deneka D, Sawicka M, Lam AK, Paulino C, Dutzler R. Structure of a volume-regulated anion channel of the LRRC8 family. Nature 2018;558:254-259.
- Sirianant L, Ousingsawat J, Wanitchakool P, Schreiber R, Kunzelmann K. Cellular volume regulation by anoctamin 6: Ca2+, phospholipase A2 and osmosensing. Pflugers Arch 2016;468:335-349.
- Sirianant L, Wanitchakool P, Ousingsawat J, Benedetto R, Zormpa A, Cabrita I, Schreiber R, Kunzelmann K. Non-essential contribution of LRRC8A to volume regulation. Pflugers Arch 2016;468:805-816.
- Akita T, Okada Y. Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the central nervous system. Neuroscience 2014;275:211-231.
- Gaitán-Peñas H, Gradogna A, Laparra-Cuervo L, Solsona C, Fernández-Dueñas V, Barrallo-Gimeno A, Ciruela F, Lakadamyali M, Pusch M, Estévez R. Investigation of LRRC8-mediated volume-regulated anion currents in
Xenopus oocytes. Biophys J 2016;111:1429-1443. - Park H, Han KS, Oh SJ, Jo S, Woo J, Yoon BE, Lee CJ. High glutamate permeability and distal localization of Best1 channel in CA1 hippocampal astrocyte. Mol Brain 2013;6:54.
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science 2010;330:790-796.
- Milenkovic A, Brandl C, Milenkovic VM, Jendryke T, Sirianant L, Wanitchakool P, Zimmermann S, Reiff CM, Horling F, Schrewe H, Schreiber R, Kunzelmann K, Wetzel CH, Weber BH. Bestrophin 1 is indispensable for volume regulation in human retinal pigment epithelium cells. Proc Natl Acad Sci U S A 2015;112:E2630-E2639.
- Fujii Y, Maekawa S, Morita M. Astrocyte calcium waves propagate proximally by gap junction and distally by extracellular diffusion of ATP released from volume-regulated anion channels. Sci Rep 2017;7:13115.
- Sabirov RZ, Okada Y. The maxi-anion channel: a classical channel playing novel roles through an unidentified molecular entity. J Physiol Sci 2009;59:3-21.
- Suzuki M. The
Drosophilatweety family: molecular candidates for large-conductance Ca2+-activated Cl− channels. Exp Physiol 2006;91:141-147. - Okada Y, Okada T, Islam MR, Sabirov RZ. Molecular identities and ATP release activities of two types of volume-regulatory anion channels, VSOR and Maxi-Cl. Curr Top Membr 2018;81:125-176.
- Jung E, Osswald M, Blaes J, Wiestler B, Sahm F, Schmenger T, Solecki G, Deumelandt K, Kurz FT, Xie R, Weil S, Heil O, Thomé C, Gömmel M, Syed M, Häring P, Huber PE, Heiland S, Platten M, von Deimling A, Wick W, Winkler F. Tweety-homolog 1 drives brain colonization of gliomas. J Neurosci 2017;37:6837-6850.
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014;34:11929-11947.
- Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY, Oh SJ, Hwang EM, Marmorstein AD, Bae YC, Park JY, Lee CJ. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 2012;151:25-40.
- Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, Jaenisch R, Jacks T. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A 2004;101:10380-10385.
- Park H, Oh SJ, Han KS, Woo DH, Park H, Mannaioni G, Traynelis SF, Lee CJ. Bestrophin-1 encodes for the Ca2+-activated anion channel in hippocampal astrocytes. J Neurosci 2009;29:13063-13073.
- Benfenati V, Nicchia GP, Svelto M, Rapisarda C, Frigeri A, Ferroni S. Functional down-regulation of volume-regulated anion channels in AQP4 knockdown cultured rat cortical astrocytes. J Neurochem 2007;100:87-104.
- Oh SJ, Lee CJ. Distribution and function of the bestrophin-1 (Best1) channel in the brain. Exp Neurobiol 2017;26:113-121.
- Marmorstein LY, Wu J, McLaughlin P, Yocom J, Karl MO, Neussert R, Wimmers S, Stanton JB, Gregg RG, Strauss O, Peachey NS, Marmorstein AD. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1). J Gen Physiol 2006;127:577-589.
- Wang R, Lu Y, Gunasekar S, Zhang Y, Benson CJ, Chapleau MW, Sah R, Abboud FM. The volume-regulated anion channel (LRRC8) in nodose neurons is sensitive to acidic pH. JCI Insight 2017;2:e90632.
- Decher N, Lang HJ, Nilius B, Brüggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of I(Cl,swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol 2001;134:1467-1479.
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl- currents in cultured rat astrocytes. J Physiol 2006;572:677-689.
- Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol 1994;267:C1203-C1209.
- He Y, Ramsay AJ, Hunt ML, Whitbread AK, Myers SA, Hooper JD. N-glycosylation analysis of the human Tweety family of putative chloride ion channels supports a pentaspanning membrane arrangement: impact of N-glycosylation on cellular processing of Tweety homologue 2 (TTYH2). Biochem J 2008;412:45-55.
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 1995;15:193-204.
- MacVicar BA, Hochman D. Imaging of synaptically evoked intrinsic optical signals in hippocampal slices. J Neurosci 1991;11:1458-1469.
- Benedetto R, Sirianant L, Pankonien I, Wanitchakool P, Ousingsawat J, Cabrita I, Schreiber R, Amaral M, Kunzelmann K. Relationship between TMEM16A/anoctamin 1 and LRRC8A. Pflugers Arch 2016;468:1751-1763.
- Pedersen SF, Kapus A, Hoffmann EK. Osmosensory mechanisms in cellular and systemic volume regulation. J Am Soc Nephrol 2011;22:1587-1597.
- Ando-Akatsuka Y, Shimizu T, Numata T, Okada Y. Involvements of the ABC protein ABCF2 and alpha-actinin-4 in regulation of cell volume and anion channels in human epithelial cells. J Cell Physiol 2012;227:3498-3510.
- Okada Y, Okada T, Sato-Numata K, Islam MR, Ando-Akatsuka Y, Numata T, Kubo M, Shimizu T, Kurbannazarova RS, Marunaka Y, Sabirov RZ. Cell volume-activated and volume-correlated anion channels in mammalian cells: their biophysical, molecular, and pharmacological properties. Pharmacol Rev 2019;71:49-88.
- Cameron PL, Ruffin JW, Bollag R, Rasmussen H, Cameron RS. Identification of caveolin and caveolin-related proteins in the brain. J Neurosci 1997;17:9520-9535.
- Stahlhut M, van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell 2000;11:325-337.
- Trouet D, Carton I, Hermans D, Droogmans G, Nilius B, Eggermont J. Inhibition of VRAC by c-Src tyrosine kinase targeted to caveolae is mediated by the Src homology domains. Am J Physiol Cell Physiol 2001;281:C248-C256.
- Browe DM, Baumgarten CM. Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J Gen Physiol 2003;122:689-702.
- Matthews CA, Shaw JE, Hooper JA, Young IG, Crouch MF, Campbell HD. Expression and evolution of the mammalian brain gene Ttyh1. J Neurochem 2007;100:693-707.
- Kleinman CL, Gerges N, Papillon-Cavanagh S, Sin-Chan P, Pramatarova A, Quang DA, Adoue V, Busche S, Caron M, Djambazian H, Bemmo A, Fontebasso AM, Spence T, Schwartzentruber J, Albrecht S, Hauser P, Garami M, Klekner A, Bognar L, Montes JL, Staffa A, Montpetit A, Berube P, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel PM, Duchaine T, Perotti C, Fleming A, Faury D, Remke M, Gallo M, Dirks P, Taylor MD, Sladek R, Pastinen T, Chan JA, Huang A, Majewski J, Jabado N. Fusion of TTYH1 with the C19MC microRNA cluster drives expression of a brain-specific DNMT3B isoform in the embryonal brain tumor ETMR. Nat Genet 2014;46:39-44.
- Wiernasz E, Kaliszewska A, Brutkowski W, Bednarczyk J, Gorniak M, Kaza B, Lukasiuk K. Ttyh1 protein is expressed in glia
in vitro and shows elevated expression in activated astrocytes following status epilepticus. Neurochem Res 2014;39:2516-2526. - Raslan A, Bhardwaj A. Medical management of cerebral edema. Neurosurg Focus 2007;22:E12.



