miR-381 Attenuates Peripheral Neuropathic Phenotype Caused by Overexpression of PMP22
Ji-Su Lee1, Geon Kwak1, Hye Jin Kim1, Hwan-Tae Park2, Byung-Ok Choi1,3*, and Young Bin Hong4*
1Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul 06351, Korea.
2Department of Physiology, College of Medicine, Dong-A University, Busan 49201, Korea.
3Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea.
4Department of Biochemistry, College of Medicine, Dong-A University, Busan 49201, Korea.
Correspondence to: *To whom correspondence should be addressed.
Young Bin Hong, TEL: 82-51-240-2762, FAX: 82-51-240-2971
e-mail: ybhong@dau.ac.kr
Byung-Ok Choi, TEL: 82-2-3410-1296, FAX: 82-2-3410-0052
e-mail: bochoi@skku.edu
Received: January 23, 2019; Revised: April 5, 2019; Accepted: April 11, 2019
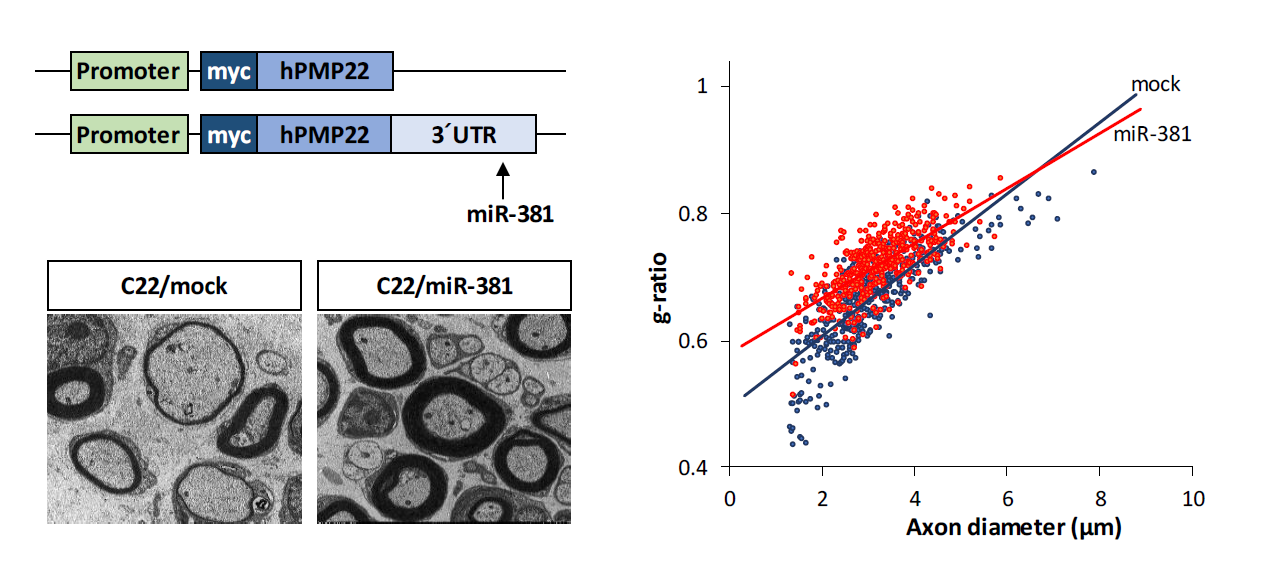
Charcot-Marie Tooth disease type 1A (CMT1A), the major type of CMT, is caused by duplication of peripheral myelin protein 22 (PMP22) gene whose overexpression causes structural and functional abnormalities in myelination. We investigated whether miRNA-mediated regulation of PMP22 expression could reduce the expression level of PMP22, thereby alleviating the demyelinating neuropathic phenotype of CMT1A. We found that several miRNAs were down-regulated in C22 mouse, a CMT1A mouse model. Among them, miR-381 could target 3′ untranslated region (3′UTR) of PMP22 in vitro based on Western botting and quantitative Real Time-PCR (qRT-PCR) results. In vivo efficacy of miR-381 was assessed by administration of LV-miR-381, an miR-381 expressing lentiviral vector, into the sciatic nerve of C22 mice by a single injection at postnatal day 6 (p6). Administration of LV-miR-381 reduced expression level of PMP22 along with elevated level of miR-381 in the sciatic nerve. Rotarod performance analysis revealed that locomotor coordination of LV-miR-381 administered C22 mice was significantly enhanced from 8 weeks post administration. Electrophysiologically, increased motor nerve conduction velocity was observed in treated mice. Histologically, toluidine blue staining and electron microscopy revealed that structural abnormalities of myelination were improved in sciatic nerves of LV-miR-381 treated mice. Therefore, delivery of miR-381 ameliorated the phenotype of peripheral neuropathy in CMT1A mouse model by down-regulating PMP22 expression. These data suggest that miRNA can be used as a potent therapeutic strategy to control diseases with copy number variations such as CMT1A.
Keywords: Peripheral myelin protein 22 (PMP22), Demyelination, microRNA (miRNA), Charcot-Marie-Tooth disease (CMT)


