Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2019; 28(3): 425-435
Published online June 26, 2019
https://doi.org/10.5607/en.2019.28.3.425
© The Korean Society for Brain and Neural Sciences
Three-axis Modification of Coordinates Enables Accurate Stereotactic Targeting in Non-human Primate Brains of Different Sizes
Hyung-Sun Kim1, Goo-Hwa Kang1, Hanlim Song2, Ra Gyung Kim2, Ji-Young Park2, Jeong Ho Hwang1, and Hyoung-Ihl Kim2,3*
1Animal model research group, Korea Institute of Toxicology, Jeongup 53212, Korea.
2Neuromodulation Lab, Department of Biomedical Science and Engineering, Gwangju Institute of Science and technology, Gwangju 61005, Korea.
3Department of Neurosurgery, Presbyterian Medical Center, Jeonju 54987, Korea.
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-62-715-3234, FAX: 82-62-715-5309
e-mail: hyoungihl@gist.ac.kr
Abstract
The brain grows with age in non-human primates (NHPs). Therefore, atlas-based stereotactic coordinates cannot be used directly to target subcortical structures if the size of the animal's brain differs from that used in the stereotactic atlas. Furthermore, growth is non-uniform across different cortical regions, making it difficult to simply apply a single brain-expansion ratio. We determined the skull reference lines that best reflect changes in brain size along the
Graphical Abstract
Keywords: Stereotaxy, Nonhuman primate, Skull, Body weight
INTRODUCTION
Experimental research in non-human primates (NHPs) is important not only for validating the concepts derived from small-animal experiments but also as a step toward possible human applications. A variety of experimental procedures are performed in NHPs that require accurate neuroanatomical targeting, including electrode placement, delivery of genes or drugs to the brain, and selective destruction of particular regions within the brain. Stereotactic techniques and instruments are commonly used to achieve such targeting in NHPs.
Stereotactic procedures require an accurate stereotactic atlas, illustrating anatomical structures in
To overcome this lack of reliability, ventriculography and magnetic resonance imaging (MRI) can be used to identify encephalic landmarks such as the anterior commissure–posterior commissure line (AC-PC line) [6,7]. Although this approach enables the correct stereotactic coordinates to be obtained with satisfactory accuracy, these techniques are not readily available to most researchers because they are complex and require costly facilities. Because these techniques exceed the capabilities of most laboratories, NHP research has generally been restricted to highly specialized laboratories. An alternative approach is to use skull landmarks derived from x-rays, on the assumption that the size of skull and the size of the brain correlate with each other with respect to body size and gender [4,8,9,10]. However, the brain and the skull do not grow symmetrically and a simple allometric calculation from body weight and skull landmarks often fails to reflect the proper growth of the brain. There is disproportionate growth of the brain and skull across the different cortical regions: the postnatal change in volume is greatest in the frontal and temporal lobes and smallest in the occipital and parietal lobes. This indicates that there is greater anterior–posterior expansion of the brain in the frontal lobe and that the inter-aural line, which lies in the EBZ plane, moves caudally as the temporal lobe grows [9,11].
To address these limitations, we performed computerized tomography (CT) and made craniometric measurements of the length of the skull reference lines and assessed how these vary with body weight. We evaluated the relative ability of the various skull reference lines to reflect the non-uniform growth of the brain along the
MATERIALS AND METHODS
All animal care and handling was in accordance with the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Korean Institute of Toxicology (KIT-1609-0339). A total of 34 male cynomolgus monkeys (
CT scans were performed with a 16-channel spiral CT scanner (Somatom Emotion, Siemens Healthcare, Munich, Germany). After induction of general anesthesia with an intramuscular injection of ketamine (5 mg/kg), the animal was placed in a prone position, the gantry was aligned with the orbitomeatal line, and the scan was performed with slice thickness of 0.6 mm. After the scan, images were reconstructed into multiplanar reconstruction (MPR) views that visualizes the brain in axial, sagittal, and coronal slices via built-in software. The raw images were exported into the Dicom viewer (RadiAnt DICOM Viewer, Medixant, Poznan, Poland) for further analysis.
To assess the allometric correlation between body weight and endocranial volume, we made longitudinal measurements of the body weight and endocranial volume of all animals. Body weight was measured regularly with a digital scale (Mattler Toledo KCC150, Manualslib, USA) from the age of 35 months up to before CT scanning. Only the animals which showed the normal growth pattern were entered in this study. To measure the endocranial volume in vivo, we exported the CT data from each animal into ImageJ (NIH, Bethesda, MD, USA) and calculated the volume from the vertex to the outer margin of the foramen magnum. Briefly, all brain images were transformed into a ‘stack’ and the scale was set. In each CT slice, a region of interest (ROI) representing the endocranial content was drawn manually. For each slice, the ROI area was multiplied by the slice thickness, and the values for all of the slices were summed to produce the total volume of the brain compartment.
Fig. 1 shows the various skull reference lines used in this study. From the reconstructed 3-D CT images, cranial landmarks including the internal auditory meatus (IAM), porion (PO), anterior clinoid process (ACP), glabella (GL), opisthocranion (OPC), infraorbitale ridge (IOR), tuberculum sellae (TS), ear-bar zero (EBZ), dorsum sellae (DS), foramen magnum (FM), and the inner skull of the vertex bone were identified, then appropriate CT planes were reconstructed so that pairs of cranial landmarks appeared in the same plane. Fifteen connecting lines (skull reference lines) were drawn between pairs of cranial landmarks and their lengths measured with computer software (RadiAnt DICOM Viewer, Medixant, Poznan, Poland). These include the inter-auricular canal line (IAL) between the two internal auricular canals , the interporion line (IPL), the glabella– tuberculum sellae line (GL-TS), the glabella–tuberculum sellae vertical line (GL-TS-VL), the glabella–dorsum sellae line (GL-DS), the glabella–dorsum sellae vertical line (GL-DS-VL), the glabella– opisthocranion line (GL-OPC), the infraorbital ridge–porion line (IOR-PO), the glabella–ear-bar zero line (GL-EBZ), the glabella–ear-bar zero vertical line (GL-EBZ-VL), the tuberculum sellae–ear-bar zero vertical line (TS-EBZ-VL), the anterior clinoid process–ear-bar zero vertical line (ACP-EBZ-VL), the tuberculum sellae–vertex vertical line (TS-VVL), the foramen magnum–vertex vertical line (FM-VVL), and the foramen magnum–tuberculum sellae–vertex vertical line (FM-TS-VVL). Among these lines, the IAL and IPL represent the variability of endocranial volume along the X-axis (Fig. 1A) whereas GL-OPC, GL-EBZ, GL-EBZ-VL, GL-DS, GL-DS-VL, TS-EBZ-VL, GL-TS, GL-TS-VL, and ACP-EBZ-VL represent the variability along the
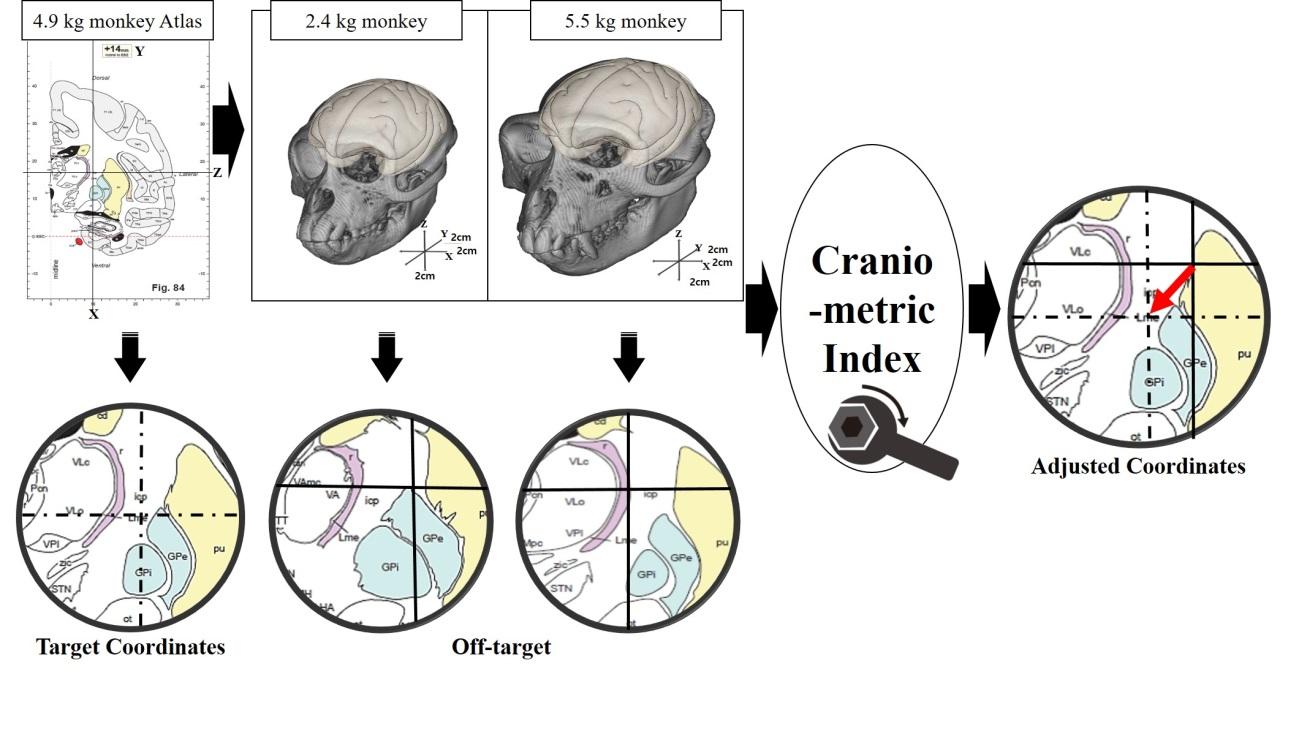
Fig. 2 shows the process by which we calculated the craniometric indices, which we used to determine accurate stereotactic coordinates in animals whose body weight did not coincide with that of the animals used in the atlases. First, we plotted the length of the skull reference lines against body weight (Fig. 3) and fit the equation
Verification of targeting was performed in three cynomolgus monkeys which were previously euthanized and preserved in compliance with the institutional guidelines of the Korean Institute of Toxicology (KIT). Three animals with body weights of 2.4 kg, 3.0 kg, and 4.1 kg were mounted in the stereotaxic apparatus. Bilateral small trephination holes were made in the skull and, after EBZ zeroing along the
To determine the statistical correlations between age, body weight, endocranial volume, and the skull reference lines, we performed a simple linear regression analysis in ORIGIN 8.5 software (OriginLab, Northampton, MA, USA). This yielded R2 values (p<0.001) and a simple regression equation for each variable such as body weight, age, and endocranial volume. We assessed all the correlations between the variables and the different skull reference lines and identified the lines that produced the greatest R2 value. An overall
RESULTS
Fig. 4 shows the relationships between body weight, age and, endocranial volume for 34 cynomolgus NHPs. All measurements are presented in Table 1. There was an overall increase in body weight with age for body weights between 2.4 kg and 5.5 kg (overall R2=0.73, p<0.0001). Beyond 60 months of age, there was no increase in body weight (Fig. 4A). Fig. 4B and 4C show the relationships between body weight or age and endocranial volume. The correlation score for the relationship between body weight and endocranial volume (R2=0.47) was statistically higher than the correlation score for the relationship between age and endocranial volume (R2=0.30).
Table 2 and Fig. 3 show the relationships between skull reference lines representing the stereotactic
The craniometric index was calculated as the ratio of the reference line length in the atlas to the reference line length derived from the simple linear regression equation. The craniometric equation was calculated separately for each axis, using the reference line with the highest R2 value for that axis. Table 3 shows the values used to calculate the craniometric indices for each axis for a 2.5 kg NHP. The craniometric index was similar for the
Modification of stereotactic targets by the axis-specific craniometric indices was validated by microinjection of methylene blue. Fig. 5 shows the dye staining after injections for multiple targets for the internal capsule, caudate & putamen and substantia nigra in animals of different body weights. For example, in the NPH with a body weight of 2.4 kg, stereotactic microinjection of methylene blue according to the atlas coordinates (
Table 4 shows a comparison of the stereotactic coordinates for the right internal capsule in a 2.5 kg NHP derived from the stereotactic atlas, together with the coordinates derived after craniometric index corrections. The
The average errors were 23% (
DISCUSSION
In this study, we identified reliable skull reference lines that can be used to adjust stereotactic coordinates to take into account disproportionate skull growth along the different axes. Among the skull reference lines, the inter-porion line (IPL) and the glabella–ear-bar zero (GL-EBZ) line had the highest R2 values for the
Stereotactic surgery in NHPs is complex and usually requires access to a costly imaging facility to accurately identify the intracerebral target. We previously designed a simplified adaptor that can be mounted on a small-animal stereotactic frame and that enables easy atlas-based stereotactic targeting of subcortical structures in NHPs without the need for high-resolution imaging [13]. However, brain size varies with sex, age, body weight, and environmental factors such as obesity, disease, etc. Because the standard stereotactic atlases are based on a brain of fixed weight, they do not provide information on how to adjust the coordinates to account for variability in brain size and weight [1]. Although this problem can be overcome with high-resolution MRI imaging, a fiducial-marker-equipped, expensive stereotactic frame, and computeraided targeting, most general laboratories do not have access to such facilities.
The stereotactic atlas provides the distances from EBZ in a NHP with fixed body weight [1,2]. When we compared the endocranial volume and skull growth with age and body weight, body weight had a stronger relationship with overall brain expansion than age. However, the brain grows disproportionately along the different axes, rotating in forward and downward directions as body weight increases [5]. Consequently, the use of a simple magnification ratio to account for brain expansion does not conform to the characteristics of patterns in brain growth [11]. We selected the skull reference lines that reflect the changes of brain size separately for the
Historically, the determination of stereotactic coordinates was based on skull reference lines [11,14,15]. However, this led to controversial results in the stereotactic targeting, and ultimately to recommendation to use the anterior commissure – posterior commissure (AC-PC) line instead [6]. Nonetheless, Wagman et al. recommend using the oblique anterior clinoid process – EBZ distance to modify the coordinates for the
This study has some limitations. First, the craniometric index corrections do not provide sub-millimeter precision. Even after applying this technique, some errors may exist. Therefore, this technique is not recommended for stereotactic targeting requiring sub-millimeter accuracy. Second, the relationship between body weight and the length of the skull reference lines is not universal. We attempted to minimize errors by excluding body weights that fell outside of the normal growth pattern and by limiting the range of body weights in the study to 2.4~5.5 kg. Within these constraints, we found a relatively strong relationship between body weight and the length of the skull reference lines. We believe that staying within these constraints is important for the accuracy of the craniometric-index modifications.
In summary, we described skull reference lines that reflect the changes in brain size with changes in body weight. Craniometric indices based on reference-line measurements can be used to adjust atlas-based coordinates so that they are appropriate for a different brain size. Combined with our previously described simple stereotactic frame adaptor, the craniometric indices enable simple, inexpensive, and accurate targeting of subcortical structures.
Figures


Tables
*p<0.05, **p<0.01.
References
- Saleem K, Logothetis N. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. 2nd ed. Cambridge, MA: Academic Press, 2016.
- Szabo J, Cowan WM. A stereotactic atlas of the brain of the cynomolgus monkey. J Comp Neurol 1984;222:265-300.
- Martin RF, Bowden DM. A stereotaxic template atlas of the macaque brain for digital imaging and quantitative neuroanatomy. Neuroimage 1996;4:119-150.
- Holt A, Cheek D, Mellits D, Hill D. Fetal and postnatal cellular growth: hormones and nutrition. New York, NY: Wiley, 1975.
- Wagman IH, Loeffler JR, McMillan JA. Relationship between growth of brain and skull of Macaca mulatta and its importance for the stereotaxic technique. Brain Behav Evol 1975;12:116-134.
- Percheron G, Yelnik J, François C. Systems of coordinates for stereotactic surgery and cerebral cartography: advantages of ventricular systems in monkeys. J Neurosci Methods 1986;17:69-88.
- Min HK, Ross EK, Lee KH, Dennis K, Han SR, Jeong JH, Marsh MP, Striemer B, Felmlee JP, Lujan JL, Goerss S, Duffy PS, Blaha C, Chang SY, Bennet KE. Subthalamic nucleus deep brain stimulation induces motor network BOLD activation: use of a high precision MRI guided stereotactic system for nonhuman primates. Brain Stimul 2014;7:603-607.
- Bronson R. Brain weight-boidy weight relationship in 12 species of nonhuman primate. Am J Phys Anthropol 1981;56:77-81.
- Scott JA, Grayson D, Fletcher E, Lee A, Bauman MD, Schumann CM, Buonocore MH, Amaral DG. Longitudinal analysis of the developing rhesus monkey brain using magnetic resonance imaging: birth to adulthood. Brain Struct Funct 2016;221:2847-2871.
- Isler K, Christopher Kirk E, Miller JM, Albrecht GA, Gelvin BR, Martin RD. Endocranial volumes of primate species: scaling analyses using a comprehensive and reliable data set. J Hum Evol 2008;55:967-978.
- Aggleton JP, Passingham RE. Stereotaxic surgery under X-ray guidance in the rhesus monkey, with special reference to the amygdala. Exp Brain Res 1981;44:271-276.
- Choi K, Chang J, Lee MJ, Wang S, In K, Galano-Tan WC, Jun S, Cho K, Hwang YH, Kim SJ, Park W. Reference values of hematology, biochemistry, and blood type in cynomolgus monkeys from cambodia origin. Lab Anim Res 2016;32:46-55.
- Kim HS, Byun D, Kim RG, Kang GH, Park JY, Yang YS, Han SC, Kim HI. Simplified adaptor for stereotactic surgery in non-human primates. J Neurosci Methods 2018;295:139-143.
- Pellegrino LJ, Cushman AJ. In: Meyers RD. Methods in psychobiology. Cambridge, MA: Academic Press, 1971; 1971. p. 67-89.
- Hamlin H, Rakic P, Yakovlev PI. Stereotactic imprecision. Confin Neurol 1965;26:426-436.



