Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2019; 28(5): 578-592
Published online October 31, 2019
https://doi.org/10.5607/en.2019.28.5.578
© The Korean Society for Brain and Neural Sciences
Modulation of Dopaminergic Neuronal Excitability by Zinc through the Regulation of Calcium-related Channels
Jihyun Noh1* and Jun-mo Chung2
1Department of Science Education, Dankook University, Yongin 16890, 2Department of Brain and Cognitive Sciences, Ewha Womans University, Seoul 03760, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-31-8005-3842, FAX: 82-31-8021-7231
e-mail: jihyun2@dankook.ac.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, andreproduction in any medium, provided the original work is properly cited.
Abstract
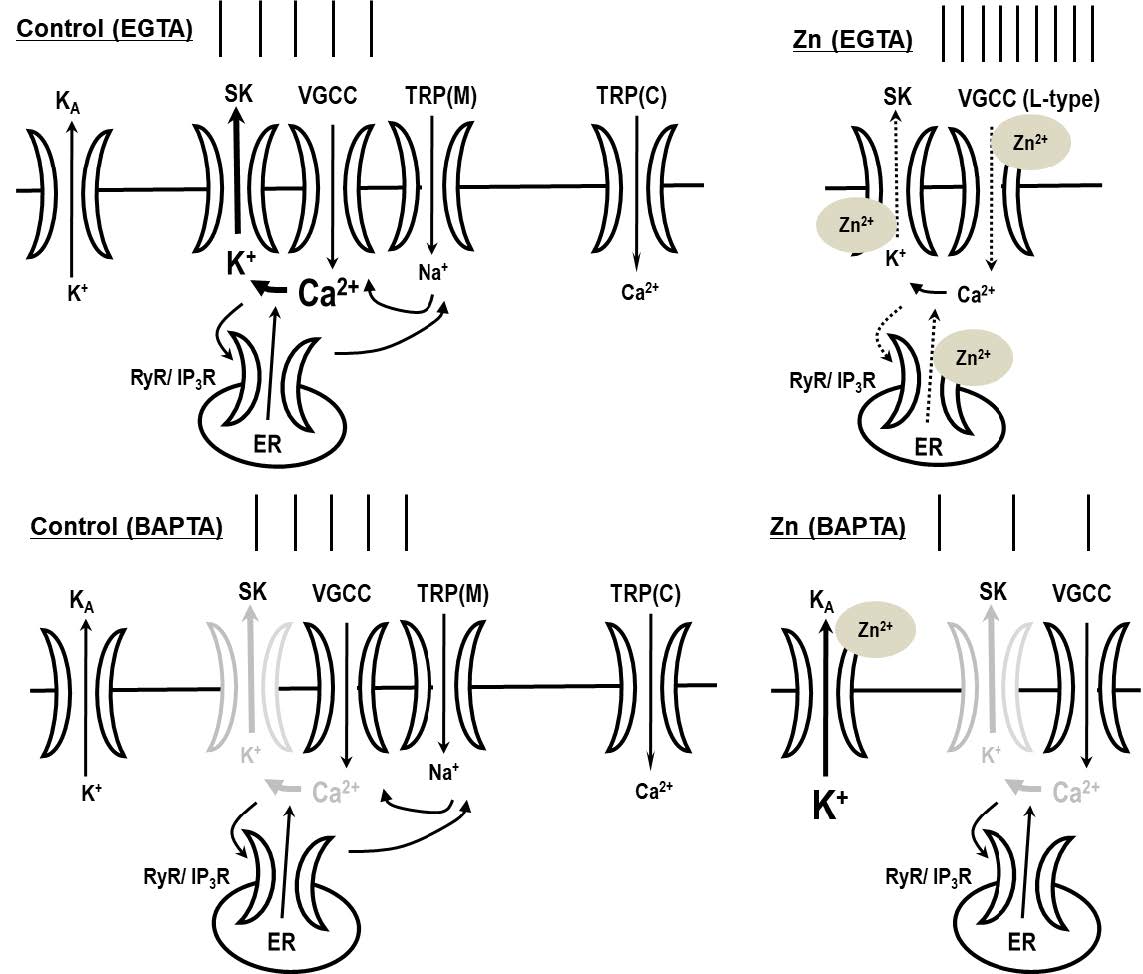
Depending on the intracellular buffering of calcium by chelation, zinc has the following two apparent effects on neuronal excitability: enhancement or reduction. Zinc increased tonic activity in the depolarized state when neurons were intracellularly dialyzed with EGTA but attenuated the neuronal activity when BAPTA was used as an intracellular calcium buffer. This suggests that neuronal excitability can be modulated by zinc, depending on the internal calcium buffering capacity. In this study, we elucidated the mechanisms of zinc-mediated alterations in neuronal excitability and determined the effect of calcium-related channels on zinc-mediated alterations in excitability. The zinc-induced augmentation of firing activity was mediated via the inhibition of small-conductance calcium-activated potassium (SK) channels with not only the contribution of voltage-gated L-type calcium channels (VGCCs) and ryanodine receptors (RyRs), but also through the activation of VGCCs via melastatin-like transient receptor potential channels. We suggest that zinc modulates the dopaminergic neuronal activity by regulating not only SK channels as calcium sensors, but also VGCCs or RyRs as calcium sources. Our results suggest that the cytosolic calcium-buffering capacity can tightly regulate zinc-induced neuronal firing patterns and that local calcium-signaling domains can determine the physiological and pathological state of synaptic activity in the dopaminergic system.
Graphical Abstract
Keywords: Calcium-activated non-selective cation, Dopamine system, Electrophysiology, Patch clamp recording, Spike frequency, Rat


