Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2023; 32(6): 387-394
Published online December 31, 2023
https://doi.org/10.5607/en23018
© The Korean Society for Brain and Neural Sciences
Distinct Role of Parvalbumin Expressing Neurons in the Reticular Thalamic Nucleus in Nociception
Sanggeon Park1,2, Jeiwon Cho1,2* and Yeowool Huh3,4*
1Department of Brain and Cognitive Sciences, Scranton College, Ewha Womans University, Seoul 03760, 2Brain Disease Research Institute, Ewha Brain Institute, Ewha Womans University, Seoul 03760, 3Department of Medical Science, College of Medicine, Catholic Kwandong University, Gangneung 25601, 4Translational Brain Research Center, International St. Mary’s Hospital, Catholic Kwandong University, Incheon 22711, Korea
Correspondence to: *To whom correspondence should be addressed.
Jeiwon Cho, TEL: 82-2-3277-4226, FAX: 82-2-3277-6595
e-mail: jelectro21@ewha.ac.kr
Yeowool Huh, TEL: 82-32-290-2773, FAX: 82-2-3277-6595
e-mail: huh06@cku.ac.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Loss of inhibition is suggested to cause pathological pain symptoms. Indeed, some human case reports suggest that lesions including the thalamic reticular nucleus (TRN) which provides major inhibitory inputs to other thalamic nuclei, may induce thalamic pain, a type of neuropathic pain. In support, recent studies demonstrated that activation of GABAergic neurons in the TRN reduces nociceptive responses in mice, reiterating the importance of the TRN in gating nociception. However, whether biochemically distinct neuronal types in the TRN differentially contribute to gating nociception has not been investigated. We, therefore, investigated whether the activity of parvalbumin (PV) and somatostatin (SOM) expressing neurons in the somatosensory TRN differentially modulate nociceptive behaviors using optogenetics and immunostaining techniques. We found that activation of PV neurons in the somatosensory TRN significantly reduced nociceptive behaviors, while activation of SOM neurons in the TRN had no such effect. Also, selective activation of PV neurons, but not SOM neurons, in the TRN activated relatively more PV neurons in the primary somatosensory cortex, which delivers inhibitory effect in the cortex, when measured with cFos and PV double staining. Results of our study suggest that PV neurons in the somatosensory TRN have a stronger influence in regulating nociception and that their activations may provide further inhibition in the somatosensory cortex by activating cortical PV neurons.
Graphical Abstract
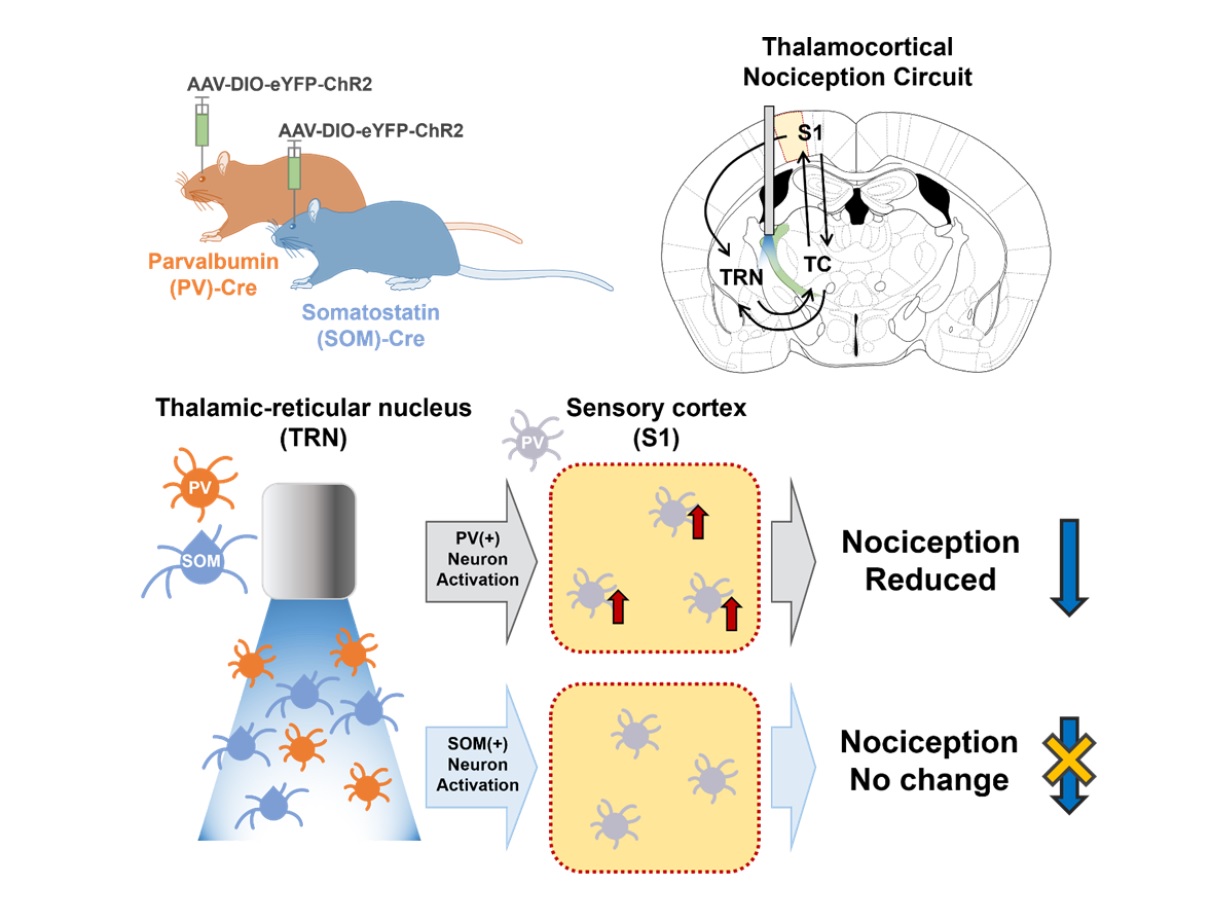
Keywords: Thalamus reticulate nucleus, Interneurons, Parvalbumin, Somatostatin, Optogenetics, Mice
INTRODUCTION
The thalamic reticular nucleus (TRN) is a structure mainly composed of GABAergic neurons that provide major inhibitory inputs to thalamic nuclei, where all sensory information except for olfaction is processed and thereby hypothesized to act as a gatekeeper of various sensory information including pain. Several clinical cases report that some brain lesions that include the TRN cause thalamic pain [1-4], which is a type of intolerable neuropathic pain. Animal studies also support that directly activating GABAergic neurons in the TRN produces an anti-nociceptive effect [5, 6].
Clinical reports and basic research results clearly support the importance of the TRN in regulating nociception. The TRN, however, is not a uniform structure, but rather a complex structure with functionally orchestrated anteroposterior and laminar connectivity patterns along with biochemically and electrophysiologically diverse cell types [7, 8]. To precisely understand how nociception is regulated, the function of microcircuits must be investigated in detail. An exemplary study that emphasizes this is the finding that activating only one type of GABAergic neuron, either parvalbumin (PV) or somatostatin (SOM) expressing interneurons, in the somatosensory cortex had an anti-nociceptive effect [9, 10] while merely activating all cortical GABAergic neurons had not [11]. At this point, whether different types of TRN neurons differentially contribute to regulating nociception has not been investigated.
Our previous study suggested that TRN neurons with different electrophysiological properties may contribute differentially to regulating nociception. Interestingly, low threshold burst firing patterns measured with slice whole patch recording of TRN PV and SOM expressing neurons [12] were similar to the “typical” and “atypical” burst firing TRN neurons, respectively, in our study [13]. Typical bursting TRN neurons are the ones that had prominent long-lasting bursts composed of many burst spikes, while atypical bursting TRN neurons are the ones that showed relatively short bursts with fewer burst spikes. The study suggests that typical bursting neurons would have a greater influence than atypical bursting neurons in regulating nociception. The differential effect of the two neuronal populations in TRN may, in part, occur by differentially affecting the primary sensory cortex (S1) because S1 is part of the thalamocortical circuit where sensory perception is thought to occur. Although molecular signatures do not necessarily coincide with the electrophysiological properties of neurons, in the present study, we investigated whether the two different types of TRN neurons, PV and SOM neurons, would differentially contribute to regulating nociception. We first investigated whether optogenetic activation of TRN PV or SOM neurons affects nociceptive behaviors in mice. After finding that they do differentially modulate nociceptive behaviors, we investigated whether the effect is related to differential activity in S1 with immunostaining of cFos proteins which is a marker of neuronal activity.
MATERIALS AND METHODS
Animals
All experiments were performed under the protocols approved by the Institutional Animal Care and Use Committee at the Catholic Kwandong University (approval number: CKU-03-2020-011). The precaution was taken to minimize the stress of animals. Either PV-Cre male mice (10~18 weeks; IMSR_JAX:008069) or SOM-Cre male mice (10~18 weeks; IMSR_JAX:017320) were used in our study. Mice were grouped-housed before surgical procedures. After surgery mice were housed individually. All mice were maintained at 12h light-dark cycle (lights on at 9 P.M.) with free access to food and water.
Surgery
Stereotaxic surgeries were performed under isoflurane anesthesia (2~2.5% for induction and 1.0~1.2% for maintenance of anesthesia) for virus injection and implantation of an optic fiber implantation into the TRN. Stereotaxic coordination was calculated based on the Paxinos and Franklin mouse brain atlas [14]. Either AAV-DIO-ChR2-eYFP or AAV-DIO-eYFP viruses (University of North Carolina Vector Core) were injected into the somatosensory TRN (AP: -1.0, ML: -1.9, DV: -2.9) of either a PV-Cre or a SOM-Cre mice with a microinjection syringe pump (World Precision Instruments). The total volume of 300 nl (flow rate: 100 nl/min) was injected into the TRN unilaterally. Then, an optic fiber (GIF 625; Thor Labs) was implanted into the right somatosensory TRN (AP: -1.0, ML: -1.9, DV: -2.8). Experiments started two weeks after the surgery.
Optogenetics and nociceptive behavior
The differential contribution of the different cellular subtypes in the TRN and nociceptive behavior was assessed by selectively activating each cellular subtype with optogenetics. Only mice that were verified to have expression in the target TRN region were used for behavioral analyses. As activating TRN PV or SOM may differentially modulate different nociceptive modalities, three behavioral tests—Electronic von Frey (mechanical threshold), Hargreaves test (thermal nociceptive threshold), and formalin tests (acute inflammatory pain)—were sequentially performed (2-week interval between different tests) with optical stimulation. Experimental procedures were carried out as explained in Huh et al. 2018 [10]. Before the start of all experiments, mice were handled and habituated to the experimental setting for 30 min/day for a week. All experiments were carried out blinded to the background of each mice (ChR2, eYFP, PV-Cre, or SOM-Cre).
Mechanical thresholds were measured with an electronic von Frey test device (IITC Inc.). The force (g) when the mouse withdrew the paw pad stimulated with a von Frey filament was recorded as the mechanical threshold. For thermal nociceptive threshold measurements, the Hargreaves test (Ugo Basil) was used. In the test, the duration each mice takes to withdraw the paw stimulated with an infrared (IR) laser is the thermal threshold. The IR laser intensity was kept constant throughout the experiment. The cut-off time (maximum length of IR stimulation) for the Hargreaves test was set at 30 s to avoid any tissue injury. For both the von Frey and Hargreaves test, two measurements were taken before (baseline) and after light stimulations in the left and right paw of each mice. Light stimulation was turned on right before a measurement was taken. All optical stimulations were 20 Hz (10 ms pulse, tip power 0.8 mW), and 473 nm blue light stimulation (Shanghai Dream Lasers), but the stimulation duration differed among different behavioral tests. The duration of each light stimulation was 10 s for the von Frey tests and 30 s for the Hargreaves test, which is an approximate time it takes to make each measurement. Also since repeated poking and heating may cause a wind-up effect (lowering of nociceptive thresholds), measurements were taken with at least 10 min intervals for the von Frey test and at least 30 min intervals for the Hargreaves test.
For the formalin test, light stimulation was turned on during the two peaks of nociceptive behaviors, the 0~5 min and the 20~30 min intervals, respectively, after a formalin injection (10 μl of 5% formalin diluted in saline) into the left hind paw. Two investigators blind to groups analyzed the videotaped behaviors of the formalin test. The duration of the licking and shacking time of the formalin-injected paw was marked and analyzed in 5 min blocks.
Immunostaining and image analysis
To investigate whether optogenetic activation of PV or SOM neurons in the TRN differentially affects the activity of the PV-expressing neurons in the primary somatosensory cortex (S1), we delivered blue light stimulation in the three groups (control (eYFP), TRN PV, TRN SOM) and labelled cFos and PV with immunostaining. Mice in all groups were habituated to the experimental setting and procedure (connecting a fiber optic patch without light stimulation) every day to minimize stress for a week before the experiment. On the day of the experiment, mice received blue light stimulation (20 Hz of 10 ms 475 nm blue light pulses for 5 min) in the TRN, deeply anesthetized with urethane (1.5 g/kg, intraperitoneal injection) about 15 min before perfusion, and transcardially perfused with saline (0.9%) followed by 10% formalin solution 90 min after blue light stimulation. Brains extracted after transcardial perfusion were fixed in 10% formalin for an hour and stored in 30% sucrose solution for a day. Fixed brain tissues were cut in coronal sections (40 μm) with a microtome (Leica). From each animal three representative brain sections with the primary somatosensory cortex corresponding to the hind limb (S1HL) region (AP: -0.10 mm, -0.58 mm, -1.06 mm) were processed for cFos and PV double fluorescence labeling. Free floating brain sections were washed in PBS for 15 min and blocked with 4% normal donkey serum containing 0.4% Triton X-100 for 2 h at room temperature. Sections were then incubated in a mixture of 1:100 mouse anti-cFos (Abcam; ab208942) and 1:1000 rabbit anti-parvalbumin (Abcam; ab11427) primary antibodies for 72 h at 4°C. Afterwards, the sections were incubated with a mixture of 1:500 AlexaFluor 568 anti-mouse (Abcam; ab175472) and 1:500 AlexaFluor 405 anti-rabbit (Abcam; ab175649) secondary antibodies for 1 h.
Images of the S1HL layers 4 and 5 regions were acquired with a fluorescent microscope (Olympus IX82). Laser settings were kept constant throughout the whole experiment. The number of cFos and PV-labeled neurons were manually counted by investigators blind to groups using the ImageJ program.
Verification of viral expressions
To verify viral expression in the TRN, mice were deeply anesthetized with urethane (1.5 g/kg, intraperitoneal) and transcardially perfused with saline (0.9%) followed by formalin (10%). Extracted brains were further fixed in formalin for an hour and stored in 30% sucrose solution for a day at room temperature. Brain tissues were then cut in coronal sections (40 μm) with a microtome (Leica). Sections containing the TRN region were examined with a fluorescent microscope (Olympus IX82) to examine the location of viral expression. Only data that were obtained from subjects that had expression in the target TRN were used in the analysis.
Statistical analysis
All data were analyzed with the SPSS 13.0 software and graphs were plotted with Microsoft Excel. Kruskal-Wallis followed by the Mann-Whitney U test was used to compare means between groups. Wilcoxon Signed Ranks test was used to compare within-group differences in behaviors before and after blue light stimulation. Repeated measures ANOVA followed by Games Howell post hoc was used to compare the difference in changes over time between groups.
RESULTS
Activation of TRN PV, but not TRN SOM, neurons modulates nociceptive behaviors
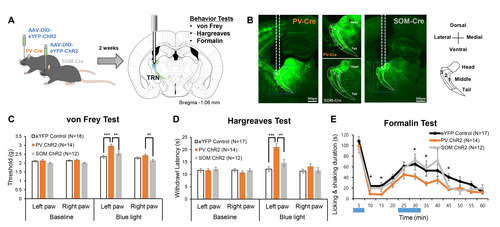
We investigated whether PV and SOM-expressing neurons in the TRN differentially regulate nociceptive behaviors with optogenetics. Fig. 1A delineates the experimental flow. PV-Cre and SOM-Cre mice were used to selectively express ChR2, an opsin that excites neurons in the presence of blue light, in respective neuronal types in the TRN. PV-Cre or Som-Cre mice that only expressed eYFP were used as controls. Two weeks after viral injection in the right TRN, mice sequentially underwent three different types of behavioral tests—von Frey test, Hargreaves test, and formalin test—with unilateral blue light stimulation. The different behavioral tests were employed to assess changes in the mechanical threshold, acute thermal threshold, and inflammatory nociception due to the activation of PV or SOM neurons in the TRN, as they may have different influences on different types of nociception. The sequence of the tests was chosen to start with a test that provides the least to the most irritant stimulation with a two-week interval between tests. Only data obtained from animals that expressed ChR2 in the target somatosensory TRN was used in the analysis (Fig. 1B). The distribution of PV and SOM neurons was consistent with previous findings [12]; PV neurons were evenly distributed throughout the TRN, while SOM neurons were sparse in layer 2 of the body part (Fig. 1B).
Selective activation of either PV or SOM neurons in the TRN revealed that only activation of the PV neurons with blue light significantly increases mechanical thresholds measured with the von Frey test (Fig. 1C, blue light). Before blue light stimulation, mechanical thresholds of both the left and right hind paw were similar among the three groups (Fig. 1C, baseline). Blue light stimulation significantly increased the left and right hind paw thresholds compared to the baseline in all groups (Fig. 1C, Wilcoxon Signed Ranks Test; baseline vs blue light; eYFP control left paw p<0.05, PV.ChR2 left paw p<0.001, SOM.ChR2 left paw p<0.001; eYFP control right paw p<0.05, PV.ChR2 right paw p<0.001, SOM.ChR2 right paw p<0.05). Activation of PV neurons with blue light also enhanced the mechanical threshold of the ipsilateral paw, the right paw, compared to the SOM-activated group. Nonetheless, the magnitude of mechanical threshold increase was greatest in the left paw of the PV-activated group, and it significantly differed from other groups (Kruskal-Wallis; H(2)=22.71, p<0.001).
Similar to changes in mechanical thresholds, only activation of TRN PV neurons significantly reduced nociceptive behaviors (Fig. 1D, 1E). Activation of PV neurons significantly increased acute heat pain thresholds measured with the Hargreaves test; withdrawal latency of both the left and right paws increased (Wilcoxon Signed Ranks Test; baseline vs blue light; PV.ChR2 left paw p<0.001, PV.ChR2 right paw p<0.001). Activation of SOM neurons tended to increase the acute heat threshold of the left paw compared to the baseline (Wilcoxon Signed Ranks Test; p=0.08), but it was insignificant. Only the heat pain threshold of the left paw of the PV-activated group significantly differed from other groups (Kruskal-Wallis; H(2)=25.24, p<0.001).
The formalin test which measures inflammatory nociception also showed that activating PV neurons in the TRN had a significant anti-nociceptive effect (Fig. 1E). Formalin (5%, 10 μl) was injected into the left paw, and the duration of licking and shaking of the inflicted paw was used to quantify the degree of nociception. Because the test lasts for an hour, blue light stimulation was given during the two peaks of a formalin test: 0~5 min and 20~30 min. Activation of PV neurons significantly reduced nociceptive behaviors in the 5~15 min, 25~30 min, and 40~45 min intervals compared to the control. Unlike PV activation, SOM activation reduced nociception compared to the control only in the 40~45 min interval. Overall, results of the behavioral tests consistently suggest that only activation of TRN PV neurons significantly modulates both the sensory and nociceptive behaviors in mice.
Activation of TRN PV neurons activates PV neurons in the primary somatosensory cortex
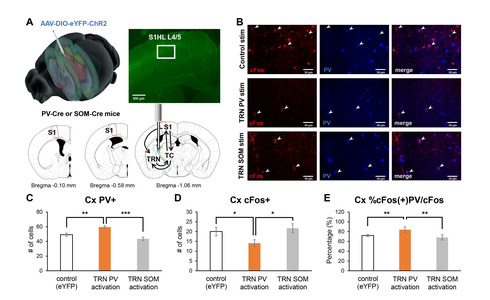
Behavioral changes induced by the activation of TRN PV or SOM neurons would have likely occurred by affecting the connected circuits. The TRN is part of the thalamocortical circuit that is composed of the thalamus and the cortex [15]. The TRN is reciprocally connected with thalamocortical (TC) neurons which send projection to the cortex (Fig. 2A). How the activity of TRN PV or SOM neurons affects the activity of thalamocortical neurons has already been investigated in another study [12], and our previous study verified that the activity of PV-expressing neurons in the primary somatosensory cortex (S1) critically involves in regulating nociception [10]. Thus, in this study, we investigated whether optogenetic regulation of the activity of TRN PV or SOM neurons differentially affects the activity of S1 PV neurons by indirectly measuring the activity of S1 PV neurons with cFos and PV immunostaining.
Specifically, we counted the number of cFos+ and PV+ neurons in the ipsilateral layer 4 of S1 corresponding to the hind limb region (S1HL L4) after activating either the TRN PV or TRN SOM neurons with blue light (Fig. 2A). We investigated L4 because it is the cortical layer that directly receives ipsilateral inputs from the TC neurons. Sample immunostaining images of the different experimental groups are shown in Fig. 2B. Analysis revealed that the TRN PV neuron activated group has the greatest number of PV+ neurons in the cortex (Fig. 2C; Kruskal-Wallis; H(2)=14.67, p<0.001) and the lowest number of cFos+ cells in the cortex (Fig. 2D; Kruskal-Wallis; H(2)=6.22, p<0.05) among the groups, suggesting that activation of TRN PV neurons significantly suppressed most cellular activities. The percentage of neurons co-expressing cFos and PV among the cFos+ cells in S1HL L4, however, was highest for the TRN PV activated group (Fig. 2E; Kruskal-Wallis; H(2)=11.96, p<0.01).
DISCUSSION
The TRN is a structure critically involved in regulating nociception. Using optogenetics, we found that, of the two different types of neurons in the somatosensory TRN, only the activity of PV neurons significantly modulated nociceptive behaviors. In addition, our study suggests that the anti-nociceptive effect induced by TRN PV activation may occur by also activating PV interneurons in the primary somatosensory cortex.
How would TRN PV neurons exert an anti-nociceptive effect?
The activity of PV neurons in the somatosensory TRN may exert an anti-nociceptive effect by regulating the thalamocortical circuit: 1) by directly inhibiting thalamocortical (TC) neurons and 2) by indirectly influencing cortical activity via changes in the activity of TC neurons.
Activation of TRN PV neurons, compared to the activation of TRN SOM neurons, was shown to induce stronger evoked inhibitory postsynaptic current (eIPSC) in the ventroposteromedial (VPM) and ventroposterolateral (VPL) nuclei [12], which are primary somatosensory relay nuclei of the thalamus that sends projection to the primary sensory cortex (S1). Greater IPSC in the thalamus induced by TRN PV neuronal activation would likely inhibit incoming nociceptive signals from being further transmitted. Furthermore, strong inhibitory input that induces robust low threshold burst firing of TC neurons [16] could provide further inhibition by recruiting inhibitory neurons in the cortex, since burst firing of TC neurons may act as an additional inhibition mechanism in the cortex by preferentially activating PV interneurons in the S1 [10]. The activity of TRN PV neurons, therefore, may produce an anti-nociceptive effect by generating more inhibition in the thalamic and in the cortex.
In support of the hypothesis, we found that optogenetic activation of the TRN PV neurons significantly enhanced the percentage of cFos+ PV cells in the S1. This effect was absent in TRN SOM activation; cFos+ cell count in the S1 induced by TRN SOM activation was not different from that of the eYFP control. Therefore, a significantly higher proportion of cells positive for both cFos and PV, along with the lowest number of cFos+ cells, may suggest that the activity of the S1 PV neurons reduced the activity of other cells in S1.
Possibility of TRN PV activation reducing cortical theta
Aberrant brain rhythms such as thalamocortical dysrhythmia are related to many neurological disorders, including implications in pathological pain [17, 18]. Patients with pathological pain tended to have increased theta and gamma rhythms compared to controls. Although thalamocortical dysrhythmia is hypothesized to occur by increased inhibition of thalamocortical neurons, factors that lead to the generation of pathological brain rhythm may be complex since lesion of the TRN which provides inhibitory inputs potentiated theta rhythms [19]. Whatever the cause, reduction of the theta rhythm seems to reduce pain in pathological and non-pathological pain conditions since activation of TRN GABAergic neurons enhanced acute thermal threshold and also reduced cortical theta rhythms [6].
Whether TRN PV neuronal activation reduces cortical theta was not directly measured in this study. However, it may exert stronger pain modulation effect than TRN SOM by modulating other pain-related brain rhythms such as the sigma band, also called the beta band. Reduced beta power was observed in chronic pain conditions [20]. TRN PV neuron activation, but not TRN SOM neuron activation, increased S1 sigma oscillation [12]. In future studies, it would be interesting to investigate how activation of TRN PV or TRN SOM neurons differentially changes S1 rhythms.
PV neurons in different TRN sectors differentially control nociception
As mentioned in the introduction, the TRN is a complex structure with functionally distinct connections. Neurons in the limbic TRN and somatosensory TRN were found to exhibit opposite activities in sleep and attentional states [21]; sensory TRN neurons were active during the awake state while limbic TRN neurons were active during sleep. Similarly, PV neurons in different TRN sectors differentially regulated nociception: while this study showed that optogenetically activating PV neurons in the somatosensory TRN increased nociceptive thresholds, chemogenetically activating PV neurons in the limbic TRN lowered both the mechanical and thermal nociceptive thresholds [22]. These findings suggest that PV neurons in different TRN sectors would play different roles in regulating nociception.
Would TRN SOM neurons have a role in gating nociception?
Activation of somatosensory TRN SOM neurons did not alter nociceptive behaviors tested in this study. In accordance, no significant cellular activity changes were observed in the S1 by activating TRN SOM neurons. At this point, there is no evidence to support that TRN SOM neuron in the somatosensory sector regulates nociceptive signals. The results, however, do not negate the possibility that TRN SOM neurons in another sector may regulate nociception. Like the activation of TRN VB neurons in the somatosensory and limbic sector which had opposite effects, the activity of TRN SOM neurons in the limbic sector may have a different role from the somatosensory sector.
Results of this study also does not rule out the possibility that TRN SOM neuron may be involved in regulating affective pain. PV and SOM neurons in the somatosensory TRN was reported to have slightly different connectivity pattern within the thalamus. While PV neurons provide inhibitory inputs mainly to the VPM, VPL, and posteromedial thalamic complex (PO), SOM neurons provide major inhibitory input to the intralaminar thalamic nucleus (IL), and weaker inhibitory input to the VPL [12]. All of these thalamic nuclei have been implicated in pain regulation, but the IL especially has been implicated in regulating affective and motivational dimensions of pain [23]. Additionally, TRN SOM neurons were also found to receive input from the central amygdala, which is a structure closely related to processing emotion [12]. Hence, activating somatosensory TRN SOM neurons may alter behavior related to affective pain.
Possible contribution of TRN in conditioned pain behaviors
Pain is a highly complex and multimodal experience. Various cognitive and emotional aspects modulate pain behaviors. Previous conditioning experience can also lead to reduced or enhanced pain response to the same stimulates, called the conditioned hypoalgesia and hyperalgesia, respectively [24]. A study demonstrated that the conditioned hypoalgesia is mediated by hippocampal NMDA receptors [25]. A nucleus in the thalamus called the reunions has connection with the hippocampus and is also innervated by TRN neurons [26]. Therefore, different TRN neurons may influence conditioned pain behaviors via the reunions.
Limitations of the study
In our study, light stimulation enhanced mechanical thresholds of both paws in all groups. This phenomenon was absent in the thermal nociception test. What caused the discrepancy is unclear. However, painfulness of a sensory input could be modulated by other sensory inputs [27]. So one reason may be that competing sensory inputs, such as the blue light stimulation used in the experiment, may have greater modulatory influence on mechanical thresholds than thermal nociceptive thresholds.
Another unresolved finding of this study is that mechanical and thermal thresholds of both paws were significantly enhanced by unilateral (right) TRN PV activation. Although the contralateral (left) paw of the TRN PV stimulated group exhibited the greatest increase, the ipsilateral (right) paw thresholds were also significantly enhanced compared to the baseline. This tendency of the TRN PV stimulation to increase the ipsilateral paw thresholds may have caused significant differences in the right paw mechanical threshold between the TRN PV and TRN SOM groups. The TRN PV activation-induced increase in the ipsilateral paw thresholds may be due to inter-hemispheric connections in the brain. The brain is a heavily interconnected structure and the somatosensory cortices are interconnected by the corpus callosum. Moreover, a recent study reports inter-hemispheric connection that goes through the thalamus [28]. Therefore, stimulation in one hemisphere may travel to the other hemisphere to produce an effect on the other side of the body.
Also, even though we tried to investigate the different effects of TRN PV or TRN SOM activation on S1 PV neuron activity with cFos assay to speculate how it may have affected nociceptive behaviors, it does not provide a direct link between the different behaviors and S1 PV neuronal activities. The cFos assay is inherently an indirect measure of neuronal activity [29] and, therefore, difficult to provide a direct relationship. In future studies, we would like to use techniques such as fiber-photometry, which are able to simultaneously measure real-time neuronal activities and behavior, combined with optogenetics to investigate a more direct relationship between S1 PV neuron activity induced by TRN or TRN SOM activation and nociceptive behaviors.
Understanding the precise microcircuit function will provide a basis for developing better pain control methods
We found that PV neurons in the somatosensory TRN modulate nociceptive behaviors in mice. Since activating PV neurons in the TRN limbic and somatosensory sectors oppositely modulate nociception, regulating the activity of all PV neurons in the TRN may not reduce nociceptive behaviors. Therefore, to effectively control pain, we should understand the working mechanism of microcircuits and be able to manipulate specific neuronal types in precise regions.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Science ICT through the National Research Foundation of Korea (NRF) grants: NRF- 2021R1C1C1006607 and NRF- 2022M3E5E8018421.
Figures
References
- Wilkins RH, Brody IA (1969) The thalamic syndrome. Arch Neurol 20:559-562
- Andy OJ (1986) Chronic pain as a reticular formation syndrome. Pavlov J Biol Sci 21:50-59
- Watson RT, Valenstein E, Heilman KM (1981) Thalamic neglect. Possible role of the medial thalamus and nucleus reticularis in behavior. Arch Neurol 38:501-506
- Vartiainen N, Perchet C, Magnin M, Creac'h C, Convers P, Nighoghossian N, Mauguière F, Peyron R, Garcia-Larrea L (2016) Thalamic pain: anatomical and physiological indices of prediction. Brain 139(Pt 3):708-722
- Zhang C, Chen RX, Zhang Y, Wang J, Liu FY, Cai J, Liao FF, Xu FQ, Yi M, Wan Y (2017) Reduced GABAergic transmission in the ventrobasal thalamus contributes to thermal hyperalgesia in chronic inflammatory pain. Sci Rep 7:41439
- LeBlanc BW, Cross B, Smith KA, Roach C, Xia J, Chao YC, Levitt J, Koyama S, Moore CI, Saab CY (2017) Thalamic bursts down-regulate cortical theta and nociceptive behavior. Sci Rep 7:2482
- Pinault D (2004) The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev 46:1-31
- Crabtree JW (2018) Functional diversity of thalamic reticular subnetworks. Front Syst Neurosci 12:41
- Cichon J, Blanck TJJ, Gan WB, Yang G (2017) Activation of cortical somatostatin interneurons prevents the development of neuropathic pain. Nat Neurosci 20:1122-1132
- Huh Y, Jung D, Seo T, Sun S, Kim SH, Rhim H, Chung S, Kim CH, Kwon Y, Bikson M, Chung YA, Kim JJ, Cho J (2018) Brain stimulation patterns emulating endogenous thalamocortical input to parvalbumin-expressing interneurons reduce nociception in mice. Brain Stimul 11:1151-1160
- Eto K, Ishibashi H, Yoshimura T, Watanabe M, Miyamoto A, Ikenaka K, Moorhouse AJ, Nabekura J (2012) Enhanced GABAergic activity in the mouse primary somatosensory cortex is insufficient to alleviate chronic pain behavior with reduced expression of neuronal potassium-chloride cotransporter. J Neurosci 32:16552-16559
- Clemente-Perez A, Makinson SR, Higashikubo B, Brovarney S, Cho FS, Urry A, Holden SS, Wimer M, Dávid C, Fenno LE, Acsády L, Deisseroth K, Paz JT (2017) Distinct thalamic reticular cell types differentially modulate normal and pathological cortical rhythms. Cell Rep 19:2130-2142
- Huh Y, Cho J (2016) Differential responses of thalamic reticular neurons to nociception in freely behaving mice. Front Behav Neurosci 10:223
- Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates. 2nd ed. Academic Press, San Diego, CA
- Jones EG (2007) The thalamus. 2nd ed. Cambridge University Press, Cambridge
- Park S, Sohn JW, Cho J, Huh Y (2019) A computational modeling reveals that strength of inhibitory input, E/I balance, and distance of excitatory input modulate thalamocortical bursting properties. Exp Neurobiol 28:568-577
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP (1999) Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A 96:15222-15227
- Walton KD, Llinás RR (2010) Central pain as a thalamocortical dysrhythmia: a thalamic efference disconnection?. In: Translational pain research: from mouse to man (Feingold KR, Anawalt B, Blackman MR edsKruger L, Light AR eds). CRC Press, Boca Raton, FL
- Marini G, Ceccarelli P, Mancia M (2002) Thalamocortical dysrhythmia and the thalamic reticular nucleus in behaving rats. Clin Neurophysiol 113:1152-1164
- Kim JA, Davis KD (2021) Neural oscillations: understanding a neural code of pain. Neuroscientist 27:544-570
- Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, Wang F, Brown EN, Wilson MA (2014) State-dependent architecture of thalamic reticular subnetworks. Cell 158:808-821
- Liu J, Zhang MQ, Wu X, Lazarus M, Cherasse Y, Yuan MY, Huang ZL, Li RX (2017) Activation of parvalbumin neurons in the rostro-dorsal sector of the thalamic reticular nucleus promotes sensitivity to pain in mice. Neuroscience 366:113-123
- Weigel R, Krauss JK (2004) Center median-parafascicular complex and pain control. Review from a neurosurgical perspective. Stereotact Funct Neurosurg 82:115-126
- Illich PA, Salinas JA, Grau JW (1991) Conditioned changes in pain reactivity: II. In search of the elusive phenomenon of conditioned hyperalgesia. Behav Neurosci 105:478-481
- Seo DO, Pang MH, Shin MS, Kim HT, Choi JS (2008) Hippocampal NMDA receptors are necessary for auditory trace fear conditioning measured with conditioned hypoalgesia in rats. Behav Brain Res 192:264-268
- McKenna JT, Vertes RP (2004) Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol 480:115-142
- Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150:971-979
- Szczupak D, Iack PM, Liu C, Tovar-Moll F, Lent R, Silva AC; IRC5 Consortium (2021) Direct interhemispheric cortical communication via thalamic commissures: a new white-matter pathway in the rodent brain. Cereb Cortex 31:4642-4651
- Chung L (2015) A brief introduction to the transduction of neural activity into Fos signal. Dev Reprod 19:61-67





