Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2011; 20(2): 85-91
Published online June 30, 2011
https://doi.org/10.5607/en.2011.20.2.85
© The Korean Society for Brain and Neural Sciences
Microarray Screening for Genes Involved in Oligodendrocyte Differentiation in the Zebrafish CNS
Ah-Young Chung1, Suhyun Kim1, Ho Kim1,Young-Ki Bae2 and Hae-Chul Park1*
1Graduate School of Medicine, Korea University, Ansan 425-707, 2Research Institute, National Cancer Center, Goyang 410-769, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-31-412-6712, FAX: 82-31-412-6729
e-mail: hcpark67@korea.ac.kr
Within the vertebrate nervous system, myelination is required for the normal function of neurons by facilitating the rapid conduction of action potentials along axons. Oligodendrocytes are glial cells which myelinate axons in the central nervous system. Disruption of myelination and remyelination failure can cause human diseases such as multiple sclerosis. Despite the importance of myelination, the molecular basis of oligodendrocyte differentiation and myelination are still poorly understood. To understand the molecular mechanisms which regulate oligodendrocyte differentiation and myelination, novel genes were identified using a microarray analysis. The analysis used oligodendrocyte lineage cells isolated from transgenic zebrafish expressing fluorescent proteins in the oligodendrocyte lineage cells. Seven genes not previously known to be involved in oligodendrocyte differentiation were identified, and their expression during oligodendrocyte development was validated.
Keywords: oligodendrocyte, differentiation, myelination, microarray, zebrafish
The insulation of axons in the vertebrate nervous system by myelin is essential for efficient axonal conduction, and is thus required for the normal function of neurons. Oligodendrocytes are glial cells which myelinate axons in the central nervous system (CNS), and are generated from oligodendrocyte progenitor cells (OPCs). In the developing spinal cord, OPCs are generated from the pMN precursor domain of the ventral spinal cord. OPCs express olig2, a basic helix-loop-helix transcription factor (Park et al., 2002b; Zhou and Anderson, 2002), and differentiate into mature oligodendrocytes after migration into the white matter of the spinal cord. The differentiation of oligodendrocytes and the myelination of axons is a highly regulated process controlled by a number of mechanisms, including extrinsic signaling pathways and intrinsic machinery.
In the CNS, myelination disruption and remyelination failure can cause demyelinating human diseases, such as multiple sclerosis (MS). Primary demyelination usually occurs by a direct insult to the oligodendrocytes, and secondary demyelination occurs as a consequence of primary axonal loss (Emery, 2010). Remyelination is the process by which new myelin sheaths are restored to demyelinated axons, enabling them to recover lost function. Although the vertebrate CNS has a very limited regeneration capacity, remyelination generally occurs efficiently. However, despite the efficiency of remyelination in experimental models and in some clinical diseases, remyelination is often inadequate in MS, the most common primary demyelinating disease (Franklin, 2002). In MS, the major cause of primary demyelination is inflammatory damage to myelin and oligodendrocytes, but the reason for the remyelination failure is largely unknown.
To understand the molecular mechanisms which control oligodendrocyte differentiation and myelination, the RNAs expressed by oligodendrocyte lineage cells in culture have been profiled using microarray technology (Dugas et al., 2006; Cahoy et al., 2008; Chen et al., 2009). These studies have revealed distinct classes of RNAs that are differentially expressed during oligodendrocyte differentiation and have led to the identification of new factors such as Gpr17, which encodes a G protein-coupled receptor that controls the timing of oligodendrocyte differentiation (Chen et al., 2009), and myelin gene regulatory factor (Mrf), which encodes a transcription factor necessary for expression of myelin genes (Cahoy et al., 2008). However, screening for genes differentially expressed in oligodendrocyte lineage cells using cultured oligodendrocyte lineage cells has limited potential to isolate genes responsible for oligodendrocyte differentiation and myelination because these processes are also controlled by extracellular ligands and molecules secreted from neurons, such as Jagged, PSA-NCAM, and LINGO-1 (Wang et al., 1998; Charles et al., 2000; Mi et al., 2005). Here, a microarray screening method was used to isolate genes involved in oligodendrocyte differentiation using oligodendrocyte lineage cells sorted from transgenic zebrafish. Oligodendrocyte lineage cells expressing enhanced green fluorescent protein (EGFP) were isolated by fluorescence activated cell sorting (FACS), and the gene expression profiles of undifferentiated OPCs and differentiated oligodendrocytes were compared. Seven genes not previously known to be involved in oligodendrocyte differentiation were identified, and their expression during oligodendrocyte development was validated.
MATERIALS AND METHODS
Zebrafish embryos used in this study were wild-type AB line,
Embryos were dechorionated and incubated in EM with histone-deacetylase inhibitor Trichostatin A (Tsai et al., 2006) at a concentration of 100 ng/ml.
Embryos were raised until appropriate stage and anesthetized with ethyl 3-aminobenzoate methanesulfonic acid on ice. A thousand embryos were chopped into several pieces and transferred into 1.5 ml eppendorf tube. Collected samples were rinsed with calcium-, magnesium-free Ringers solution for 20 min, then washed with Dulbecco's phosphate buffered saline (D-PBS) (GIBCO) three times. Tissue was digested with 100 µl of D-PBS containing 35 µl of Liberase Blendzyme 3, followed by incubation at 29℃ for 15 min with occasional mixing. After incubation, 1 ml of trypsin (GIBCO) was added and incubated at 29℃ for 15 min until complete dissociation. To inactivate trypsin, we added 2 ml of D-PBS containing 10% fetal bovine serum (FBS) and filtered the solution through a cell strainer (BD Falcon) to remove undissociated cell clusters. We collected dissociated cells by centrifugation at 300 g at 4℃ for 5 min and rinsed twice with D-PBS containing 10% FBS. Dissociated cells were collected by centrifugation and resuspended in PBS containing 1 mM EDTA, 25 mM HEPES (pH 7.0) and 1% FBS. Cell suspensions were separated into GFP+ and GFP- cells by BD FACSAria II (Becton Dickinson) performed at room temperature under sterile conditions. Isolated GFP+ and GFP- cells were collected in D-PBS/10% FBS pre-coated tubes with RNase inhibitor.
Isolated cells were collected by low-speed centrifugation and homogenized in TRIzol solution (Invitrogen) subsequently. Total RNA extraction was performed as described previously (Peterson and Freeman, 2009). Isolated total RNA was cleaned up with RNeasy midi kit (Quiagen) and qualified as a microarray target samples using Bioanalyzer 2100 system (Agilent) and NanoDrop spectrophotometer.
1 µg of qualified total RNA was amplified and labeled with Cy3, Cy5 dyes. Hybridization was performed with Zebrafish Agilent Gene Expression Microarray Chips (Agilent), followed by washing step. Hybridized chips were then scanned, normalized and analyzed.
cDNA was synthesized using ImProm-II reverse transcription system (Promega). The quantitative real-time PCR was performed using the Lightcycler system (Roche Applied Science) with the Lightcycler-FastStart DNA Master SYBR Green I (Roche Applied Science) according to the manufacturer's instructions. Each reaction was performed in a volume of 20 µl with final concentration of 1×Lightcycler-FastStart DNA Master SYBR Green 1, 3 mM MgCl2, 0.5 µM of each primer and 2 µl of first strand cDNA mixture. Quantitative real-time PCR was carried out for 40 cycles and fluorescence readings were acquired at the end of each amplification cycle at 72℃. Melting curve analysis was performed with continuous fluorescence acquisition from 65 to 95℃ at a temperature transition rate of 0.1℃/s to determine the amplification specificity. All reactions were performed as technical duplicates.
Embryos were anesthetized until movement had ceased and fixed in 4% paraformaldehyde overnight. Fixed embryos were embedded in 1.5% agar with 5% sucrose blocks and equilibrated by 30% sucrose solution. Frozen blocks were sliced into 10 µm sections on glass slides using cryostat microtome. Sections were rinsed with PBS several times and then blocked in 2% bovine serum albumin with sheep serum. Embryos were processed for immunohistochemistry, following primary antibodies were used: rabbit anti-Sox10 (1 : 250), rabbit anti-MBP (1 : 100), rabbit anti-GFP (1 : 500, Abcam) and mouse anti-HuC/D (16A11 1 : 20, Molecular Probes, Eugene, OR). For fluorescent detection of antibody labeling, we used Alexa Fluor 568-conjugated goat anti-mouse IgG, Alexa Fluor 568-conjugated goat anti-rabbit IgG, and Alexa Fluor 488-conjugated goat anti-rabbit IgG (1 : 500, Molecular Probes). Photos were taken using a confocal lasers canning microscope (LSM 510 Pascal, Carl Zeiss).
RESULTS AND DISCUSSION
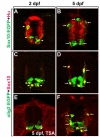
To identify the genes involved in oligodendrocyte differentiation and myelination, we isolated OPCs and differentiated oligodendrocytes expressing EGFP from the transgenic zebrafish and compared their transcriptional profiles. Therefore, the embryo stages suitable for collecting OPCs and differentiated oligodendrocytes were first identified. Previously, we have shown that
Expression of EGFP in OPCs and mature oligodendrocytes at several developmental stages in the
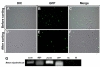
To identify the genes specific to oligodendrocytes, a comparison of transcriptional profiles of normal and oligodendrocyte-deficient larvae could be conducted. Previously, oligodendrocyte development was shown to require histone deacetylase (HDAC) activity (Marin-Husstege et al., 2002; Cunliffe and Casaccia-Bonnefil, 2006; Ye et al., 2009), and treatment with the HDAC inhibitor Trichostatin A can block formation of oligodendrocytes in zebrafish (Tsai et al., 2006). Consistent with previous reports, we found that treatment of
On the basis of the above data, OPCs and mature oligodendrocytes were purified from the dissociated cells of
To compare the transcriptional profiles of OPCs and mature oligodendrocytes, we purified RNA from the sorted cells and synthesized dye-labeled cDNAs, which were hybridized to microarray gene chips. A total of 19,956 transcripts were examined with the Agilent zebrafish gene expression microarray. Genes up-regulated by 2.5-fold in mature oligodendrocytes were identified as genes required for oligodendrocyte maturation and genes down-regulated to 0.3-fold or less in mature oligodendrocytes were identified as genes required for the maintenance of OPC status. Fig. 3A and B illustrate diagrammatically the 4348 up-regulated and 1808 down-regulated genes in mature oligodendrocytes compared to OPCs as analyzed by three different algorithms. In addition, a total of 32 up-regulated and 19 down-regulated genes were selected from the intersection of the three algorithms (Fig. 3A, B). To confirm the accuracy of the microarray screen, we searched the expression profile of well-characterized genes required for the oligodendrocyte differentiation, such as
To validate the microarray data, we performed a qRT-PCR analysis of selected genes which exhibited highly elevated expression levels in the differentiated oligodendrocytes. We selected seven genes not previously known to be involved in oligodendrocyte differentiation. The expression levels for
Rho-GTPase family proteins play important roles in coordinating the remodeling of the actin cytoskeleton during myelination. A key effecter of Rho-GTPase is Rho-kinase (ROCK), a serine/threonine kinase that regulates cell migration, proliferation and survival (Mueller et al., 2005). ROCK is expressed in Schwann cells at the onset of myelination, and pharmacological inhibition of ROCK activity causes Schwann cells form aberrant short myelin segments. However, after ROCK inhibition is removed, new myelin segments are formed normally (Melendez-Vasquez et al., 2004), indicating that ROCK plays an important role together with Rho-GTPase in the progression of the Schwann cell membrane encasement of the axon during myelination.
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 1994;120:1097-1108.
- Bolis A, Coviello S, Visigalli I, Taveggia C, Bachi A, Chishti AH, Hanada T, Quattrini A, Previtali SC, Biffi A, Bolino A. Dlg1, Sec8, and Mtmr2 regulate membrane homeostasis in Schwann cell myelination. J Neurosci 2009;29:8858-8870.
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 2008;28:264-278.
- Carney TJ, Dutton KA, Greenhill E, Delfino-Machin M, Dufourcq P, Blader P, Kelsh RN. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development 2006;133:4619-4630.
- Charles P, Hernandez MP, Stankoff B, Aigrot MS, Colin C, Rougon G, Zalc B, Lubetzki C. Negative regulation of central nervous system myelination by poly-sialylated-neural cell adhesion molecule. Proc Natl Acad Sci USA 2000;97:7585-7590.
- Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA, Trapp BD, Karandikar NJ, Hsieh J, Lu QR. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci 2009;12:1398-1406.
- Cunliffe VT, Casaccia-Bonnefil P. Histone deacetylase 1 is essential for oligodendrocyte specification in the zebrafish CNS. Mech Dev 2006;123:24-30.
- Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. Functional genomic analysis of oligodendrocyte differentiation. J Neurosci 2006;26:10967-10983.
- Eccleston PA, Silberberg DH. The differentiation of oligodendrocytes in a serum-free hormone-supplemented medium. Brain Res 1984;318:1-9.
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science 2010;330:779-782.
- Franklin RJ. Why does remyelination fail in multiple sclerosis?. Nat Rev Neurosci 2002;3:705-714.
- Gorczyca D, Ashley J, Speese S, Gherbesi N, Thomas U, Gundelfinger E, Gramates LS, Budnik V. Postsynaptic membrane addition depends on the Discs-Large-interacting t-SNARE Gtaxin. J Neurosci 2007;27:1033-1044.
- Gu J, Royland JE, Wiggins RC, Konat GW. Selenium is required for normal upregulation of myelin genes in differentiating oligodendrocytes. J Neurosci Res 1997;47:626-635.
- Kessaris N, Pringle N, Richardson WD. Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philos Trans R Soc Lond B Biol Sci 2008;363:71-85.
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253-310.
- Lee OK, Frese KK, James JS, Chadda D, Chen ZH, Javier RT, Cho KO. Discs-Large and Strabismus are functionally linked to plasma membrane formation. Nat Cell Biol 2003;5:987-993.
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci 2002;22:10333-10345.
- Melendez-Vasquez CV, Einheber S, Salzer JL. Rho kinase regulates schwann cell myelination and formation of associated axonal domains. J Neurosci 2004;24:3953-3963.
- Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy JM, Pepinsky RB. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci 2005;8:745-751.
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 2005;4:387-398.
- Park H, Mehta A, Richardson JS, Appel B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Developmental Biology 2002a;248:356-368.
- Park H, Shin J, Appel B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development 2004;131:5959-5969.
- Park HC, Appel B. Delta-Notch signaling regulates oligodendrocyte specification. Development 2003;130:3747-3755.
- Park HC, Mehta A, Richardson JS, Appel B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev Biol 2002b;248:356-368.
- Peterson SM, Freeman JL. RNA isolation from embryonic zebrafish and cDNA synthesis for gene expression analysis. J Vis Exp 2009;pii:1470
- Preusser M, Birner P, Ambros IM, Ambros PF, Budka H, Harris AL, Hainfellner JA. DEC1 expression in 1p-aberrant oligodendroglial neoplasms. Histol Histopathol 2005;20:1173-1177.
- Shin J, Park HC, Topczewska JM, Mawdsley DJ, Appel B. Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci 2003;25:7-14.
- Sun H, Taneja R.
Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. Proc Natl Acad Sci USA 2000;97:4058-4063. - Tsai HH, Macklin WB, Miller RH. Netrin-1 is required for the normal development of spinal cord oligodendrocytes. J Neurosci 2006;26:1913-1922.
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 1998;21:63-75.
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci 2009;12:829-838.
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 2002;109:61-73.