Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2019; 28(5): 593-601
Published online October 31, 2019
https://doi.org/10.5607/en.2019.28.5.593
© The Korean Society for Brain and Neural Sciences
3-Carene, a Phytoncide from Pine Tree Has a Sleep-enhancing Effect by Targeting the GABAA-benzodiazepine Receptors
Junsung Woo1, Hyejin Yang2, Minseok Yoon2, Changdev G. Gadhe3, Ae Nim Pae3*, Suengmok Cho4* and C. Justin Lee1,5*
1Center for Neuroscience, Korea Institute of Science and Technology (KIST), Department of Neuroscience, Division of Bio- Medical Science & Technology, KIST School, Korea University of Science and Technology, Seoul 02792, 2Research Division of Food Functionality, Korea Food Research Institute, Wanju 55365, 3Convergence Research Center for Diagnosis, Treatment and Care System of Dementia, Brain Science Institute, Korea Institute of Science and Technology (KIST), Seoul 02791, 4Department of Food Science and Technology, Pukyong National University, Busan 48513, 5Center for Cognition and Sociality, Institute for Basic Science, Daejeon 34126, Korea
Correspondence to: *To whom correspondence should be addressed.
Ae Nim Pae, TEL: 82-2-958-5185, FAX: 82-2-958-6999
e-mail: anpae@kist.re.kr
Suengmok Cho, TEL: 82-51-629-5833, FAX: 82-51-629-5824
e-mail: scho@pknu.ac.kr
C. Justin Lee, TEL: 82-42-878-9155, FAX: 82-42-878-9151
e-mail: cjl@ibs.re.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, andreproduction in any medium, provided the original work is properly cited.
Abstract
3-Carene, a bicyclic monoterpene, is one of the major components of the pine tree essential oils. It has been reported that, in addition to its known properties as a phytoncide, 3-carene has anti-inflammatory, antimicrobial, and anxiolytic effects. We have previously demonstrated that α-pinene, the major component of pine tree, has a hypnotic effect through GABAA-benzodiazepine (BZD) receptors. However, a hypnotic effect of 3-carene has not been studied yet. Here, we report that oral administration of 3-carene increases the sleep duration and reduces sleep latency in pentobarbital-induced sleep test. 3-Carene potentiates the GABAA receptor-mediated synaptic responses by prolonging the decay time constant of inhibitory synaptic responses. These enhancing effects of 3-carene are reproduced by zolpidem, a modulator for GABAA-BZD receptor, and fully inhibited by flumazenil, an antagonist for GABAA-BZD receptor. The molecular docking of 3-carene to the BZD site of GABAA protein structure, suggests that 3-carene binds to the BZD site of α1 and ϒ2 subunits of GABAA-BZD receptor. These results indicate that, similar to α-pinene, 3-carene shows a sleep-enhancing effect by acting as a positive modulator for GABAA-BZD receptor.
Graphical Abstract
Keywords: 3-carene, Sleep, GABAA-BZD receptor, Phytoncide
INTRODUCTION
Sleep is defined as a naturally recurring state of mind and body, characterized by altered consciousness [1], and is divided into two types: non-rapid eye movement (non-REM or NREM) and rapid eye movement (REM) sleep. Sleep is observed in non-human animals including mammals, birds, reptiles, amphibians, fish, even in insects [2–5] and is required for living animals to live and maintain a normal life. It has been growing numbers of sleep disorders: One fourth of the general population in America have experienced insomnia, one of the most common sleep disorders [6]. Although there have been various ways to treat insomnia such as behavioral therapy, psychotherapy, and light therapy, the most common one is pharmacological treatments using hypnotic drugs targeting GABAA-BZD receptors such as diazepam, zolpidem [7]. However, it has been reported that those drugs have many side effects including cognitive impairment, tolerance, headache, nausea, and rebound insomnia [8, 9]. Therefore, there has been growing need for developing new hypnotic drugs derived from natural products with less side effects.
Pines are conifer in the genus
3-Carene (3,7,7-Trimethylbicyclo[4.1.0]hept-3-ene) is a bicyclic monoterpene consisting of fused cyclohexene and cyclopropane rings and has a sweet and pungent odor [19]. It has been used as a raw material in perfumes, cosmetics, flavors and terpene resins, having various therapeutic properties including anti-inflammatory, anti-fungal, and sedative effects [20, 21]. 3-Carene is the second abundant monoterpene of pine essential oils after α-pinene. Recently we have reported a hypnotic effect of α-pinene as a positive modulator of GABAA-BZD receptors [22]. Although the beneficial effects and biological properties of 3-carene as a phytoncide of the pine tree have been established, the potential effect of 3-carene on sleep has not been tested yet. In this study, we investigated the hypnotic effect of 3-carene in mice by oral administration method. We also identified the molecular mechanism of 3-carene through the various experimental approaches including electrophysiology, pharmacological tools, and molecular modeling. Through our previous and current study, we suggest that phytoncides from pine trees including α-pinene and 3-carene commonly have sleep-enhancing effect as a positive modulator of GABAA-BZD receptors.
MATERIALS AND METHODS
Materials
3-Carene (CAS no. 13466-78-9) was purchased from Sigma Aldrich Inc. (St. Louis, MO, USA). Zolpidem (Ministry of Food and Drug Safety, Cheongwon-gun, Chungcheongbuk-do, Korea), a GABAA-benzodiazepine (BZD) receptor agonist, was used as a reference hypnotic drug. Flumazenil, an antagonist of GABAA-BZD receptors, was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Molecular structures and weights of 3-carene, zolpidem, and flumazenil are shown in Fig. 1. All other chemicals and reagents were of the highest grade available.
Animals
All procedures involving animals were conducted in accordance with the animal care and use guidelines of the Korea Food Research Institutional Animal Care and Use Committee (permission number: KFRI-M-12027). Imprinting control region (ICR; male, 18~22 g) and C57BL/6N (male 27~30 g) mice were purchased from Koatech Animal Inc. (Pyeongtaek, Korea). The animals were housed in an insulated, sound-proof recording room maintained at an ambient temperature of 23±0.5°C, with a constant relative humidity (55±2%) on an automatically controlled 12 h light/12 h dark cycle (lights off at 17:00). They had free access to food and water. All efforts were made to minimize animal suffering and to use only the number of animals required for the production of reliable scientific data.
Pentobarbital-induced sleep test
The initial screening for hypnotic effect of 3-carene sleep was done with pentobarbital-induced sleep [23]. Experiment was performed between 13:00 and 17:00 h, and the ICR mice were fasted for 24 h before the experiment to minimize the drowsiness induced by food. 3-Carene and zolpidem were administered orally (p.o.) to the ICR mice (n=10) 45 min before the pentobarbital injection (45 mg/kg, i.p.). After the injection intraperitoneally (i.p.) of pentobarbital, mice were placed in individual cages and observed for measurements of sleep latency and duration. The observers were blinded to the individual treatments. The mice were considered asleep if stayed immobile and lost its righting reflex when positioned on its back. The sleep latency was defined as the elapsed time from pentobarbital injection to onset of righting reflex loss. The sleep duration was defined as the difference in time between the loss and the recovery of the righting reflex.
Pharmacological treatments
3-Carene was dissolved in sterile saline containing 5% tween 80 immediately before use, and administered orally (p.o.) to the C57BL/6N mice (each group, n=8) at 17:00 h on the experimental day at a dose of 12.5, 25, 50, or 100 mg/kg. The positive control zolpidem (10 mg/kg) was administered in the same manner as 3-carene. Flumazenil was dissolved in sterile saline and injected intraperitoneally (i.p.) 15 min before 3-carene or zolpidem administration. For baseline data, mice were injected with the vehicle (saline containing 5% tween 80) at 16:45 h (i.p.) and 17:00 h (p.o.).
Electrophysiological measurement
Adult mice (7~9 weeks) were deeply anaesthetized until cessation of breathing and subsequently decapitated. The brain was rapidly removed and submerged in an ice-cold oxygenated artificial cerebrospinal fluid (ACSF) composed of (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1 CaCl2, 3 MgCl2, 10 glucose at pH 7.4, and was bubbled with 5% CO2/95% O2. Transverse mouse brain slices (300 μm) containing hippocampus were acutely prepared with a vibratome (Linear slicer, DSK, Japan), and incubated in a chamber with oxygenated ACSF at room temperature for 1 h before use.
The standard ACSF recording solution was composed of (mM): 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1.5 CaCl2, 1.5 MgCl2 and 10 glucose saturated with 95% O2–5% CO2, at pH 7.4. The internal solution was composed of (mM): 140 CsCl, 10 EGTA, 10 HEPES, 4 Mg-ATP, 10 QX-314. To block the spontaneous EPSC, APV (50 μM; Tocris) and CNQX (20 μM; Tocris) were added into ACSF. Recordings were obtained using Axopatch 200A (Axon instruments, CA, USA) and filtered at 2 kHz. In case of sIPSC recording, recordings were digitized at 10 kHz, and analyzed using pCLAMP 10 (Molecular devices, CA, USA) and Mini Analysis Program (Synaptosoft, NJ, USA). The sIPSCs were automatically detected. All experimental procedures described were performed in accordance with the institutional guidelines of Korea Institute of Science and Technology (KIST, Seoul, Korea).
Molecular modeling
In this study, we used previously published model for the most abundant α1β2γ2 subtype of GABAA receptor [22]. 3D coordinates of 3-carene was obtained from the PubChem database (
Data analysis
All data were expressed as the mean±SEM. Statistical analysis was performed with the SigmaPlot 13.0 (Systat Software Inc.). For multiple comparisons, data were analyzed using one-way ANOVA followed by Dunnett’s test. Comparisons between two-group data were analyzed by the unpaired Student’s t-test. The significance level was set at p<0.05 for all statistical tests.
RESULTS
Effects of 3-carene in the pentobarbital-induced sleep test
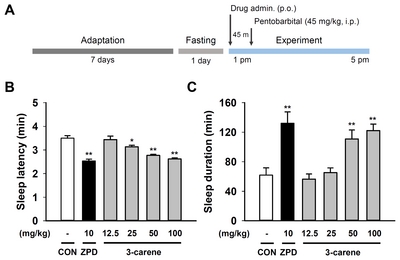
To test whether oral administration (p.o.) of 3-carene (Fig. 1) produces a sleep-enhancing effect, we performed the pentobarbital-induced sleep test in ICR mice (Fig. 2A). As expected, zolpidem (10 mg/kg, p.o.), a well-known hypnotic drug and positive modulator of GABAA-BZD receptors [25, 26], significantly decreased the sleep latency and increased the sleep duration (sleep latency: 2.53±0.08 min; sleep duration: 132.1±15.48, Fig. 2B and 2C). We found that 3-carene (100 mg/kg, p.o.) also showed a similar effect in sleep latency and duration in a dose-dependent manner (sleep latency: 2.62±0.05 min; sleep duration: 122.2±8.74, Fig. 2B and 2C) as the zolpidem.
Molecular mechanism of sleep-enhancing effect of 3-carene
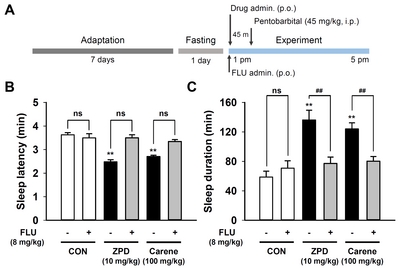
Recently we have reported that α-pinene, a monoterpene from pine essential oil, acts as a positive modulator of GABAA-BZD receptor, similarly to other monoterpenes such as borneol, verbenol, and pinocarveol [22, 27, 28]. To confirm 3-carene functions like these monoterpene, we treated flumazenil, an antagonist of GABAA-BZD receptor, 15 min before administration of 3-carene, and then measured the sleep behavior. Flumazenil (1 mg/kg) inhibited the hypnotic effect of zolpidem without affecting the sleep latency and duration (Fig. 3B and 3C). The hypnotic effect of 3-carene was fully antagonized by flumazenil, suggesting that 3-carene acts as a positive modulator of GABAA-BZD receptor, just like zolpidem.
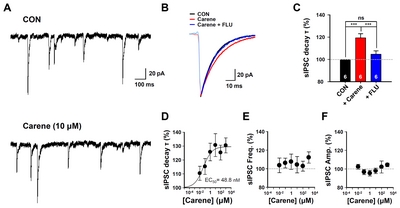
To confirm that 3-carene is a genuine positive modulator of GABAA-BZD receptor, we performed the conventional slice whole-cell patch clamp experiments. We measured the inhibitory synaptic responses from hippocampal CA1 neurons that express GABAA receptors containing α1 and α2 subunits, the well-known targets for BZD drugs such as diazepam and zolpidem [29, 30]. We found that 3-carene prolonged the decay time constant of spontaneous inhibitory postsynaptic currents (sIPSCs) in dose-dependent manner (EC50=48.8 nM, Fig. 4B and 4D). This enhancing effect in decay time by 3-carene was fully inhibited by flumazenil (1 μM, Fig. 4B and 4C). There was no significant difference in frequency and amplitude of sIPSCs (Fig. 4E and 4F), consistent with previous reports [22, 31, 32]. These data suggest that 3-carene enhances the GABAergic synaptic responses by targeting the GABAA-BZD receptor and prolonging the decay time of sIPSCs.
Docking model of 3-carene in BZD binding site of GABAA receptor
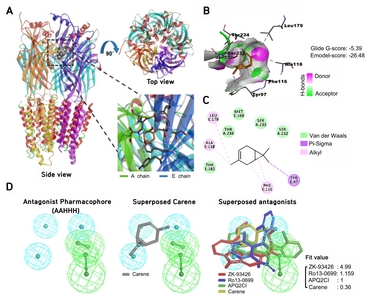
Based on the data showing 3-carene acting as a positive modulator of GABAA-BZD receptor, we predicted the binding modes of 3-carene at the BZD binding site in GABAA receptor through the docking and pharmacophore mapping. We found that 3-carene binds at BZD site of α1 and γ2 subunits of GABAA receptor with −5.39 kcal/mol glide Gscore energy (Fig. 5A and 5B). Docked pose reveals that ligand docked in the deep crevice and formed van der Waals contact with Ser232, Ser233, Thr234 of α1-subunit, and Met168 and Thr181 from γ2-subunit. Phe116 and Tyr97 forms pi-pi stacking and pi-sigma interactions with the cyclic ring, and 7-methyl, respectively (Fig. 5B). This observation coincides with the previous report about the importance of these residues in ligand interaction identified by modeling and mutagenesis [22, 33–35]. Also, 3-methyl forms alkyl-alkyl interactions with Ala118 and Leu179 of α1- and γ2-subunits, respectively (Fig. 5C). To identify the pharmacological class of 3-carene, we mapped 3-carene over developed pharmacophore models of agonist, partial agonist, and antagonist. We observed that 3-carene only mapped over an antagonist pharmacophore generated by using ZK-93426, APQ2Cl, and Ro13-0699 (Fig. 5D). In fact, 3-carene mapped over three hydrophobic features but missed two hydrogen bond acceptor function. These results indicate that 3-carene positively modulates the biological function of GABAA receptor by directly binding at the BZD binding site.
DISCUSSION
In this study, we have demonstrated that 3-carene has a sleep-enhancing effect by prolonging the decay time of GABAergic synaptic response as a positive modulator for GABAA-BZD receptor. Although it has been reported that 3-carene has been used as raw material in perfumes, cosmetics, flavors, and terpene resins with various beneficial effects such as anti-inflammatory, anti-fungal, and sedative effects [20, 21], any hypnotic effect of 3-carene has not been tested. This is the first study to identify the effect of orally administered 3-carene on sleep via the pentobarbital-induced sleep test and its molecular mechanism.
Nowadays there has been growing interest in forest bathing, defined as making contact with and taking in the atmosphere of the forest, for its various therapeutic effects such as enhancing immune system function, ameliorating respiratory and cardiovascular system, relieving stress and anxiety [36, 37]. It has been accepted that the various beneficial effects of the forest are due to plant-derived products or phytoncide, defined as natural volatile compounds derived from trees and plants [37, 38]. The ancient Chinese and Korean medicinal literatures have described the therapeutic effects of pine tree seeds in digestive function, stress, and sleep and usage of pine tree seeds in symptoms or diseases relating to stress and sleep [39, 40]. Based on these lines of ancient evidence, we demonstrate the hypnotic effect of phytoncide from pine tree and its molecular mechanism through the combination of experimental methods including sleep behavior analysis, electrophysiology, and molecular modeling.
We have previously reported that certain purified compounds from rice, marine plant, and pine tree all enhance the GABAA receptor-mediated synaptic response by prolonging the decay time constant of inhibitory synaptic response by targeting GABAA-BZD receptor [22, 23, 32, 41]. We have shown that these compounds have hypnotic effects with enhanced quantity of non-rapid eye movement sleep (NREMS) [22, 23, 32, 41]. In the current study, we found the same effects of 3-carene in IPSCs and sleep. Based on these findings, we can make a generalization that if a certain drug or compound enhances the decay time constant of IPSCs, we can safely predict that it could have a hypnotic effect as a positive modulator of GABAA-BZD receptor. Therefore, we propose that this electrophysiological assay as a simple screening method for finding a potential sleep-enhancing drug.
In this study, we tested the sleep-enhancing effect of 3-carene through pentobarbital-induced sleep test. This method serves as a screening tool for finding potential hypnotics and determining the proper concentration of drugs for further experiments such as electroencephalography (EEG) and electromyography (EMG) to examine the effect of 3-carene in sleep stages such as REM and non-REM. Therefore, without detailed sleep stage analysis with EEG and EMG, there might be a limitation to conclude that 3-carene has an effect on sleep-wake behavior and sleep architecture based only on pentobarbital-induced sleep test. However, in our previous report on α-pinene [22], we performed all the critical experiments including the EEG/EMG and detailed sleep stage analysis for α-pinene. In the same previous report, we have established that combination of pentobarbital-induced sleep test, IPSC recordings, and molecular docking are sufficient to ensure a positive effect on sleep stages. Therefore, in the current study with 3-carene, we did not perform the detailed sleep stage analysis using EEG and EMG, which takes a lot of effort and time. Instead, we believe that the pentobarbital-induced sleep test, IPSC recordings, and molecular docking are sufficient to ensure a positive effect of 3-carene on sleep. Nevertheless, to elucidate the effect of 3-carene in sleep stages and architecture, further experiments of EEG and EMG with 3-carene are needed in the future.
Here we have performed the IPSC recordings from hippocampal CA1 neurons. The rationale for performing the IPSC recordings in hippocampus is that although hippocampus is not the sleep control center, it is possible to test the general action of 3-carene in hippocampal CA1 neurons expressing GABAA receptors containing α1 and α2 subunits. These GABAA subunits are the known molecular targets for BZD drugs such as diazepam and zolpidem [29, 30]. Hippocampal CA1 pyramidal neurons also express GABAA receptors containing α1 and α2 subunits [29], just as the ventrolateral preoptic nucleus in the hypothalamus of the actual sleep center [42, 43]. The effect of BZD drugs has been tested in neurons from various brain regions including thalamus, hippocampus, and neocortex [13, 31, 44]. In our previous studies, we have tested the effect of various natural compounds on the IPSC decay kinetics in hippocampal CA1 neurons and found that the compounds also have hypnotic effects [22, 32]. Therefore, testing the effect of candidate compounds in the hippocampus CA1 is a convenient and simple way for pre-screening for potential hypnotic drugs.
In summary, we have demonstrated that 3-carene as a phytoncide from pine tree has the sleep-enhancing effect by targeting GABAA-BZD receptor by combining sleep behavior analysis, electrophysiology, and molecular modeling. In addition to the hypnotic effect of 3-carene, it is known to have other therapeutic effects such as anti-inflammatory, anti-oxidant, and anti-stress. We can easily obtain these beneficial effects of 3-carene from forest bathing, inhalation of pine essential oils, and oral supplements. We propose 3-carene as an excellent therapeutic agent for patients having sleep disorders or anxiety.
ACKNOWLEDGEMENTS
This study was supported by grants from the Korea Food Research Institute (E0164503-01) (S.C.), Creative Research Initiative Program, Korean National Research Foundation (2015R1A3A2066619) and KIST Institutional Grant (2E26662) (C.J.L.).
Figures
References
- Ferri R, Manconi M, Plazzi G, Bruni O, Vandi S, Montagna P, Ferini-Strambi L, Zucconi M (2008) A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. J Sleep Res 17:89-100.
- Allison T, Cicchetti DV (1976) Sleep in mammals: ecological and constitutional correlates. Science 194:732-734.
- Ramón F, Hernández-Falcón J, Nguyen B, Bullock TH (2004) Slow wave sleep in crayfish. Proc Natl Acad Sci U S A 101:11857-11861.
- Flanigan WF Jr (1973) Sleep and wakefulness in iguanid lizards, Ctenosaura pectinata and Iguana iguana. Brain Behav Evol 8:401-436.
- Rattenborg NC (2006) Evolution of slow-wave sleep and palliopallial connectivity in mammals and birds: a hypothesis. Brain Res Bull 69:20-29.
- University of Pennsylvania School of Medicine (2018) One in four Americans develop insomnia each year: 75 percent of those with insomnia recover [Internet]. Sciencedaily, Rockvile, MD. Available from: www.sciencedaily.com/releases/2018/06/180605154114.htm
- Gooneratne NS, Vitiello MV (2014) Sleep in older adults: normative changes, sleep disorders, and treatment options. Clin Geriatr Med 30:591-627.
- Atkin T, Comai S, Gobbi G (2018) Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev 70:197-245.
- Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN (2017) Adverse effects of hypnotic medications. J Clin Sleep Med 13:839.
- Ioannou E, Koutsaviti A, Tzakou O, Roussis V (2014) The genus Pinus : a comparative study on the needle essential oil composition of 46 pine species. Phytochem Rev 13:741-768.
- Judzentiene A, Kupcinskiene E (2008) Chemical composition on essential oils from needles of Pinus sylvestris L. grown in Northern Lithuania. J Essent Oil Res 20:26-29.
- Lee JG, Lee CG, Kwag JJ, Buglass AJ, Lee GH (2005) Determination of optimum conditions for the analysis of volatile components in pine needles by double-shot pyrolysisgas chromatography-mass spectrometry. J Chromatogr A 1089:227-234.
- Yang X, Zhao HT, Wang J, Meng Q, Zhang H, Yao L, Zhang YC, Dong AJ, Ma Y, Wang ZY, Xu DC, Ding Y (2010) Chemical composition and antioxidant activity of essential oil of pine cones of Pinus armandii from the Southwest region of China. J Med Plants Res 4:1668-1672.
- Hmamouchi M, Hamamouchi J, Zouhdi M, Bessiere JM (2001) Chemical and antimicrobial properties of essential oils of five moroccan pinaceae. J Essent Oil Res 13:298-302.
- Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils--a review. Food Chem Toxicol 46:446-475.
- Li Q (2010) Effect of forest bathing trips on human immune function. Environ Health Prev Med 15:9-17.
- Xie Q, Liu Z, Li Z (2015) Chemical composition and antioxidant activity of essential oil of six pinus taxa native to China. Molecules 20:9380-9392.
- Süntar I, Tumen I, Ustün O, Keleş H, Akkol EK (2012) Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J Ethnopharmacol 139:533-540.
- Chipman-Shlaes N (2007) The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Choice 44:1724-1726.
- Ocete MA, Risco S, Zarzuelo A, Jimenez J (1989) Pharmacological activity of the essential oil of Bupleurum gibraltaricum: anti-inflammatory activity and effects on isolated rat uteri. J Ethnopharmacol 25:305-313.
- Gil ML, Jimenez J, Ocete MA, Zarzuelo A, Cabo MM (1989) Comparative study of different essential oils of Bupleurum gibraltaricum Lamarck. Pharmazie 44:284-287.
- Yang H, Woo J, Pae AN, Um MY, Cho NC, Park KD, Yoon M, Kim J, Lee CJ, Cho S (2016) α-Pinene, a major constituent of pine tree oils, enhances non-rapid eye movement sleep in mice through GABAA-benzodiazepine receptors. Mol Pharmacol 90:530-539.
- Cho S, Kim S, Jin Z, Yang H, Han D, Baek NI, Jo J, Cho CW, Park JH, Shimizu M, Jin YH (2011) Isoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABAA receptors and shows hypnotic effects. Biochem Biophys Res Commun 413:637-642.
- Zhang W, Koehler KF, Zhang P, Cook JM (1995) Development of a comprehensive pharmacophore model for the benzodiazepine receptor. Drug Des Discov 12:193-248.
- Salvà P, Costa J (1995) Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet 29:142-153.
- Sancar F, Ericksen SS, Kucken AM, Teissére JA, Czajkowski C (2007) Structural determinants for high-affinity zolpidem binding to GABA-A receptors. Mol Pharmacol 71:38-46.
- Granger RE, Campbell EL, Johnston GA (2005) (+)- and (-)-borneol: efficacious positive modulators of GABA action at human recombinant alpha1beta2gamma2L GABA(A) receptors. Biochem Pharmacol 69:1101-1111.
- Kessler A, Sahin-Nadeem H, Lummis SC, Weigel I, Pischetsrieder M, Buettner A, Villmann C (2014) GABA(A) receptor modulation by terpenoids from Sideritis extracts. Mol Nutr Food Res 58:851-862.
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W (1996) The gamma 2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha 1 and beta 2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology 35:1425-1444.
- Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12:1040-1062.
- Perrais D, Ropert N (1999) Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci 19:578-588.
- Woo J, Cho S, Lee CJ (2014) Isoliquiritigenin, a chalcone compound, enhances spontaneous inhibitory postsynaptic response. Exp Neurobiol 23:163-168.
- Amin J, Brooks-Kayal A, Weiss DS (1997) Two tyrosine residues on the alpha subunit are crucial for benzodiazepine binding and allosteric modulation of gamma-aminobutyric acidA receptors. Mol Pharmacol 51:833-841.
- Buhr A, Baur R, Sigel E (1997) Subtle changes in residue 77 of the gamma subunit of alpha1beta2gamma2 GABAA receptors drastically alter the affinity for ligands of the benzodiazepine binding site. J Biol Chem 272:11799-11804.
- Wieland HA, Lüddens H, Seeburg PH (1992) A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem 267:1426-1429.
- Park BJ, Tsunetsugu Y, Kasetani T, Hirano H, Kagawa T, Sato M, Miyazaki Y (2007) Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest)--using salivary cortisol and cerebral activity as indicators. J Physiol Anthropol 26:123-128.
- Park BJ, Tsunetsugu Y, Kasetani T, Kagawa T, Miyazaki Y (2010) The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): evidence from field experiments in 24 forests across Japan. Environ Health Prev Med 15:18-26.
- Abe T, Hisama M, Tanimoto S, Shibayama H, Mihara Y, Nomura M (2008) Antioxidant effects and antimicrobial activites of phytoncide. Biocontrol Sci 13:23-27.
- Heo J (1980) Donguibogam. pp 1, 69, 82, 104, 597, 601. Namsandang, Seoul.
- Li S, Luo X (1975) Compendium of materia medica: bencao gangmu. Renmin weisheng Press, Beijing.
- Cho S, Yoon M, Pae AN, Jin YH, Cho NC, Takata Y, Urade Y, Kim S, Kim JS, Yang H, Kim J, Kim J, Han JK, Shimizu M, Huang ZL (2014) Marine polyphenol phlorotannins promote non-rapid eye movement sleep in mice via the benzodiazepine site of the GABAA receptor. Psychopharmacology (Berl) 231:2825-2837.
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB (1996) Activation of ventrolateral preoptic neurons during sleep. Science 271:216-219.
- Lu J, Greco MA, Shiromani P, Saper CB (2000) Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci 20:3830-3842.
- Bacci A, Rudolph U, Huguenard JR, Prince DA (2003) Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci 23:9664-9674.





