Articles
Article Tools
Supplementary
Stats or Metrics
Article
Special Topic
Exp Neurobiol 2021; 30(1): 13-31
Published online February 8, 2021
https://doi.org/10.5607/en20063
© The Korean Society for Brain and Neural Sciences
Ultimate COVID-19 Detection Protocol Based on Saliva Sampling and qRT-PCR with Risk Probability Assessment
Joungha Won1,2, Hasan Hüseyin Kazan3, Jea Kwon2,4, Myungsun Park2, Mehmet Ali Ergun5, Sureyya Ozcan6, Byung Yoon Choi7, Won Do Heo1* and C. Justin Lee2*
1Department of Biological Sciences, Korea Advanced Institute of Science and Technology, Daejeon 34141, 2Center for Cognition and Sociality, Cognitive Glioscience Group, Institute for Basic Science, Daejeon 34126, Korea, 3Department of Biological Sciences, Middle East Technical University, Ankara 06800, Turkey, 4KU-KIST Graduate School of Converging Science and Technology, Korea University, Seoul 02841, Korea, 5Department of Medical Genetics, Gazi University Faculty of Medicine, Ankara 06560, 6Department of Chemistry, Middle East Technical University, Ankara 06800, Turkey, 7Department of Otorhinolaryngology, Seoul National University Bundang Hospital, Seongnam 13620, Korea
Correspondence to: *To whom correspondence should be addressed.
Won Do Heo, TEL: 82-42-350-2642, FAX: 82-42-350-2610
e-mail: wondo@kaist.ac.kr
C. Justin Lee, TEL: 82-42-878-9150, FAX: 82-42-878-9151
e-mail: cjl@ibs.re.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
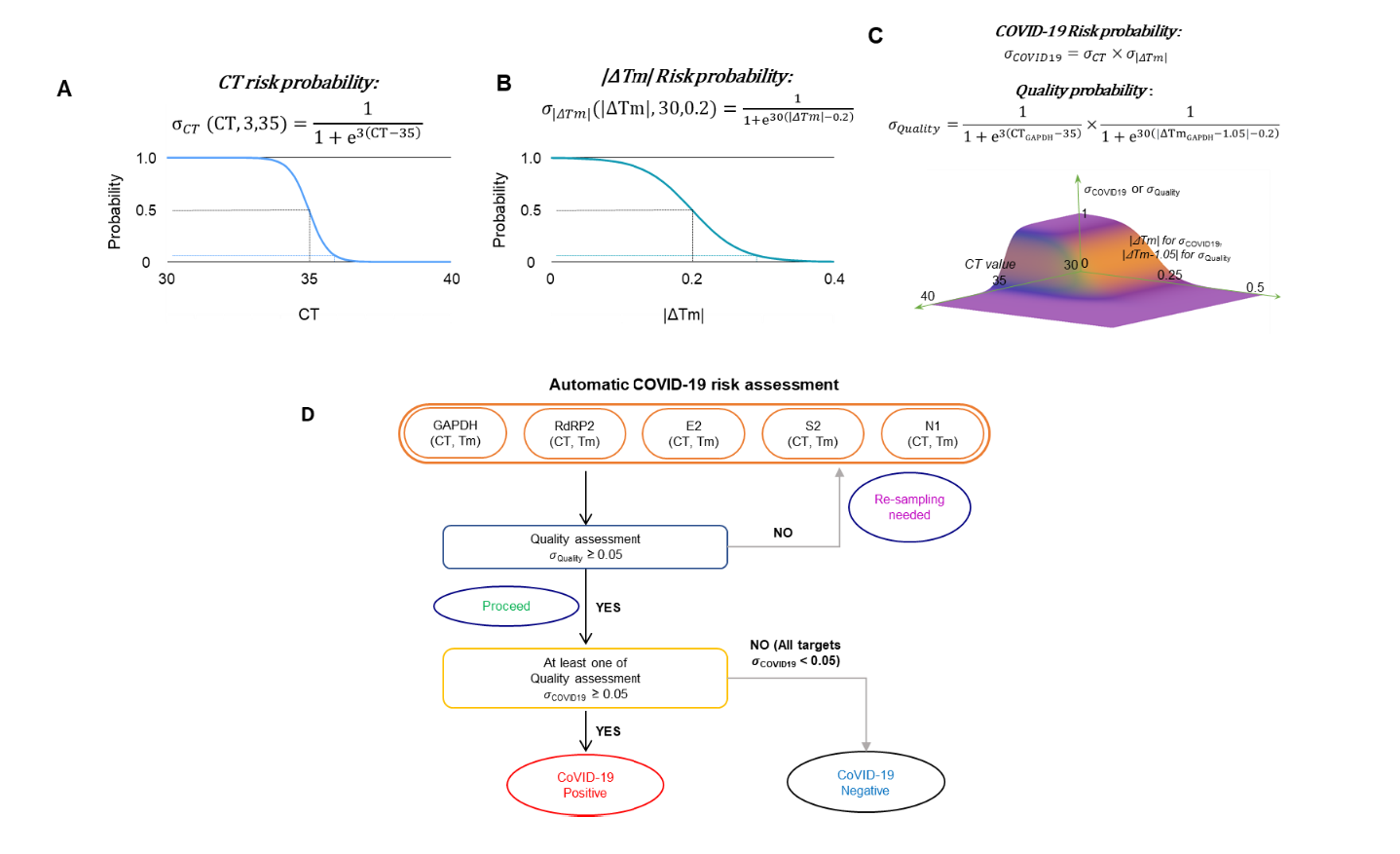
In the era of COVID-19 outbreak, various efforts are undertaken to develop a quick, easy, inexpensive, and accurate way for diagnosis. Although many commercial diagnostic kits are available, detailed scientific evaluation is lacking, making the public vulnerable to fear of false-positive results. Moreover, current tissue sampling method from respiratory tract requires personal contact of medical staff with a potential asymptomatic SARSCOV-2 carrier and calls for safe and less invasive sampling method. Here, we have developed a convenient detection protocol for SARS-COV-2 based on a non-invasive saliva self-sampling method by extending our previous studies on development of a laboratory-safe and low-cost detection protocol based on qRT-PCR. We tested and compared various self-sampling methods of self-pharyngeal swab and self-saliva sampling from non-carrier volunteers. We found that the self-saliva sampling procedure gave expected negative results from all of the non-carrier volunteers within 2 hours, indicating cost-effectiveness, speed and reliability of the saliva-based method. For an automated assessment of the sampling quality and degree of positivity for COVID-19, we developed scalable formulae based on a logistic classification model using both cycle threshold and melting temperature from the qRT-PCR results. Our newly developed protocol will allow easy sampling and spatial-separation between patient and experimenter for guaranteed safety. Furthermore, our newly established risk assessment formula can be applied to a large-scale diagnosis in health institutions and agencies around the world.
Graphical Abstract
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Saliva sampling, COVID-19 risk assessment


