Articles
Article Tools
Supplementary
Stats or Metrics
Article
Short Communication
Exp Neurobiol 2022; 31(5): 277-288
Published online October 31, 2022
https://doi.org/10.5607/en22038
© The Korean Society for Brain and Neural Sciences
Glutamate Permeability of Chicken Best1
Jung Moo Lee1,2†, Changdev Gorakshnath Gadhe3†, Hyunji Kang2,4†, Ae Nim Pae3,5* and C. Justin Lee1,2,4*
1KU-KIST Graduate School of Converging Science and Technology, Korea University, Seoul 02841, 2Center for Cognition and Sociality, Institute for Basic Science, Daejeon 34126, 3Brain Science Institute, Korea Institute of Science and Technology, Seoul 02792, 4IBS School, University of Science and Technology, Daejeon 34113, 5KIST School, University of Science and Technology, Seoul 02792, Korea
Correspondence to: *To whom correspondence should be addressed.
Ae Nim Pae, TEL: 82-2-958-5185, FAX: 82-2-958-6999
e-mail: anpae@kist.re.kr
C. Justin Lee, TEL: 82-42-878-9150, FAX: 82-42-878-9151
e-mail: cjl@ibs.re.kr
†These authors contributed equally to this article.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Bestrophin-1 (Best1) is a calcium (Ca2+)-activated chloride (Cl-) channel which has a phylogenetically conserved channel structure with an aperture and neck in the ion-conducting pathway. Mammalian mouse Best1 (mBest1) has been known to have a permeability for large organic anions including gluconate, glutamate, and D-serine, in addition to several small monovalent anions, such as Cl‑, bromine (Br-), iodine (I-), and thiocyanate (SCN-). However, it is still unclear whether non-mammalian Best1 has a glutamate permeability through the ion-conducting pathway. Here, we report that chicken Best1 (cBest1) is permeable to glutamate in a Ca2+-dependent manner. The molecular docking and molecular dynamics simulation showed a glutamate binding at the aperture and neck of cBest1 and a glutamate permeation through the ion-conducting pore, respectively. Moreover, through electrophysiological recordings, we calculated the permeability ratio of glutamate to Cl- (PGlutamate/PCl) as 0.28 based on the reversal potential shift by ion substitution from Cl- to glutamate in the internal solution. Finally, we directly detected the Ca2+-dependent glutamate release through cBest1 using the ultrasensitive two-cell sniffer patch technique. Our results propose that Best1 homologs from non-mammalian (cBest1) to mammalian (mBest1) have a conserved permeability for glutamate.
Graphical Abstract
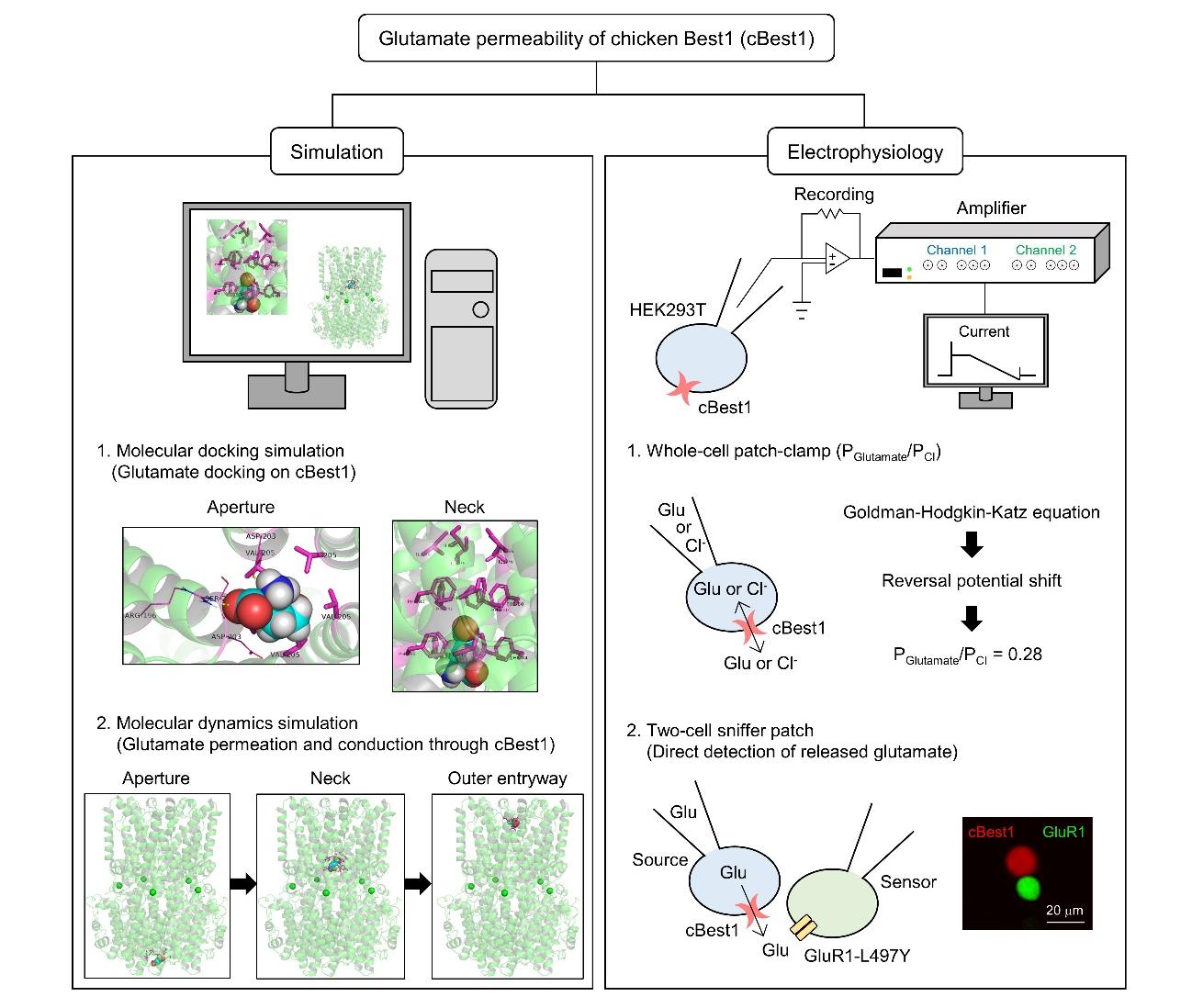
Keywords: Chicken Best1, Glutamate permeability, Molecular docking simulation, Molecular dynamics simulation, Whole-cell patch-clamp recording, Two-cell sniffer patch


