Articles
Article Tools
Stats or Metrics
Article
Review Article
Exp Neurobiol 2014; 23(2): 115-123
Published online June 30, 2014
https://doi.org/10.5607/en.2014.23.2.115
© The Korean Society for Brain and Neural Sciences
Delayed and Prolonged Local Brain Hypothermia Combined with Decompressive Craniectomy: A Novel Therapeutic Strategy That Modulates Glial Dynamics
Jong-Heon Kim1, Sung-Ho Yun2, Kwang-Ho Jang2, Jaechan Park3, Hyung Soo Han4, Dongick Rhee5 and Kyoungho Suk1*
1Department of Pharmacology, Brain Science & Engineering Institute, BK21 Plus KNU Biomedical Convergence Program, Kyungpook National University School of Medicine, Daegu 700-422, 2Department of Surgery, College of Veterinary Medicine, Kyungpook National University, Daegu 702-701, 3Department of Neurosurgery, Kyungpook National University School of Medicine, Daegu 700-721, 4Department of Physiology, Kyungpook National University School of Medicine, Daegu 700-422, 5Kyungwon Medical Co., Ltd., Seoul 135-080, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-53-420-4835, FAX: 82-53-256-1566
e-mail: ksuk@knu.ac.kr
Abstract
Hypothermia is considered a useful intervention for limiting pathophysiological changes after brain injury. Local hypothermia is a relatively safe and convenient intervention that circumvents many of the complications associated with systemic hypothermia. However, successful hypothermia treatment requires careful consideration of several factors including its practicality, feasibility, and associated risks. Here, we review the protective effects-and the cellular mechanisms that underlie them-of delayed and prolonged local hypothermia in rodent and canine brain injury models. The data show that the protective effects of therapeutic hypothermia, which mainly result from the modulation of inflammatory glial dynamics, are limited. We argue that decompressive craniectomy can be used to overcome the limitations of local brain hypothermia without causing histological abnormalities or other detrimental effects to the cooled area. Therefore, delayed and prolonged local brain hypothermia at the site of craniectomy is a promising intervention that may prove effective in the clinical setting.
Keywords: hypothermia, stroke, traumatic brain injury, astrocyte, microglia, neuroinflammation
INTRODUCTION
Induced hypothermia is widely used as first-line treatment for damaged tissues in a variety of circumstances such as fever and burn injury. Historically, Hippocrates first described the beneficial effects of hypothermia, and Napoleon's surgeons noted that wounded soldiers who stayed away from the campfire had a higher survival rate than those who stayed close. The first clinical use of mild to moderate therapeutic hypothermia (32~35℃) was reported in 1940, and the use of profound hypothermia (<30℃) for head injury was first described in 1964 [1]. However, the clinical application of therapeutic hypothermia was restricted because of systemic complications and detrimental side effects including shivering, altered clearance of medications, abnormal EKG patterns [2], arrhythmias [3], infection [4], coagulopathy [5], electrolyte disorders [6], and insulin resistance [7]. Recently, clinical reports of the benefits of therapeutic hypothermia have been accumulating, and new protocols have been developed to minimize these side effects. For example, state-of-the-art hypothermic techniques use local or regional cooling to minimize systemic side effects. Despite the fact that therapeutic hypothermia is considered an effective intervention, we still do not have a clear understanding of its underlying molecular mechanisms, nor have we been able to set therapeutic standards for its parameters such as optimal duration, target temperature, initiation time, and rates of cooling and rewarming.
In general, a brain injury is categorized as either a traumatic brain injury (TBI) or an acquired brain injury (ABI). TBI refers to an injury to the brain caused by an external force. Common causes of TBI include gunshot wounds, motor vehicle crashes, assaults, or strikes to the head from falling. ABI is a broader category that refers both to traumatic brain injuries and to brain injuries caused by internal forces such as a stroke, cerebral hemorrhage, or brain tumor. Brain injuries, caused by both external and internal forces, trigger a complex cascade of post-injury events that lead to pathophysiology. These events include failed cellular energetics leading to acidosis and the production of oxygen free radicals, excitotoxicity due to excessive glutamate signaling and increased intracellular free Ca2+, and increased cytokines/chemokines and the initiation of the inflammatory response [8]. Thus, pathophysiology in the brain is a secondary injury that develops as a consequence of a myriad of cellular and molecular mechanisms initiated after the primary injury.
In our previous studies, we designed an improved hypothermic conditioning protocol to treat rat models of TBI including stab wound [9] and transient middle cerebral artery occlusion (tMCAO) [10]. To avoid the side effects of systemic hypothermia, we performed local brain hypothermia with delayed initiation and long-term application. Our data showed that delayed local hypothermia can induce beneficial glial function and improves neurological outcomes as well as neuronal survival. We observed that after a focal stab wound, local hypothermia inhibited microglial activation and migration around the site of injury. In the tMCAO model, proteomic data indicated that glutamate signaling, cell survival, oxidative stress response, and mitochondrial function were reserved in astrocytes by local hypothermia. These data indicate that local hypothermia can produce a favorable neurological outcome by modulating glia.
We also discuss many of the other current methods and devices being used to produce local brain hypothermia including surface cooling, intranasal selective hypothermia, transarterial or transvenous endovascular cooling, extraluminal vascular cooling, and epidural cerebral cooling. Although many investigators have postulated that local brain hypothermia should have advantages over systemic hypothermia, its benefits are still being tested in animal models as well as in preclinical trials, where a clear analysis of its risk-benefit profile is currently lacking. This review will highlight the effects of optimized local brain hypothermia on brain injury and its prospective mechanisms such as a glial modulation.
LOCAL HYPOTHERMIC INTERVENTIONS
Cranial surface cooling is the most convenient and simplest cooling method. It was originally performed with ice packs or fanning. Subsequently, a cooling helmet was made using technology developed by NASA [11]. The study was designed as a randomized trial with eight patients in the experimental group and six in the control group. Brain temperature was monitored 0.8 cm below the cortical surface for 48 to 72 hours, and local hypothermia was initiated within 24 hours after stroke. Brain cooling to approximately 35℃ (2℃ below normal body temperature) was achieved within 15 minutes, and brain temperature was maintained thereafter. Core temperature did not drop below 37℃ until 4 to 5 hours later, during the 48-hour challenge. When brain cooling was stopped, the temperature of the brain approached the temperature of the core within 1 to 2 hours. There were no deleterious effects such as rebound hyperthermia, intracranial pressure (ICP) elevation, arrhythmia, infection, or coagulopathy. Further investigation implied that selective brain cooling improved the good neurological outcome score (GNOS, score 4~5) as well as the survival rate after brain injury [12, 13].
Since then, intranasal cooling methods have been developed in animal models and tested during clinical trials [14, 15]. BeneChill, Inc., developed the RhinoChill System, an intranasal device that sprays an inert evaporative coolant onto the surfaces of the upper airways and the base of the skull using a nasal prong. The major advantage of the Rhinochill system is its rapid reduction of brain temperature. In preliminary safety data from brain-injured patients, the RhinoChill device appeared safe, and it effectively lowered brain and core temperatures [14]. The study enrolled a total of 15 patients (9 in the hypothermic group and 6 in the control group) who were injured by intracerebral hemorrhage, trauma, and ischemic stroke, in equal numbers. It showed that core temperature, brain temperature, and tympanic temperature were reduced an average of 1.1±0.6℃ (range, 0.3~2.1℃), 1.4±0.4℃ (range, 0.8~5.1℃), and 2.2±2℃ (range, 0.5~6.5℃), respectively. No adverse events or complications were observed. However, keeping intranasal temperature about 2℃ lower produced unexpected side effects, and discomfort was reported. Moreover, application of intranasal cooling in the presence of skull base fractures is contraindicated for patients. Other local hypothermic trials including endovascular, extravascular, and epidural cooling remain in the experimental phase. Despite presumed advantages such as safety, convenience, rapid cooling, and reduced risk of systemic hypothermia, the practicality and feasibility of local brain hypothermia have not been fully defined. In addition, certain risks cannot yet be excluded. For example, incidence of systemic hypothermia and hyperthermic rebound should be considered when administering long-term local hypothermic treatment.
NOVEL STRATEGY FOR LOCAL BRAIN HYPOTHERMIA
Clark et al. performed selective brain hypothermia on rats using a metal cooling coil (diameter 8 mm, thick 2 mm) that they previously had developed [16]. As opposed to cranial cooling method, the use of cooling coil only reduced brain temperature in the ipsilateral striatal area. During brain cooling, no marked changes were observed in physiological parameters including core temperature, heart rate, and blood pressure. The device also led to improved outcomes in the rat models of permanent MCAO (pMCAO) [17] and intracerebral hemorrhage (ICH) [18]. In the pMCAO model, they found that prolonged cooling, even when its initiation was delayed, provided enduring behavioral and histological protection. In the ICH model, selective brain cooling attenuated brain edema but failed to provide protection. These results suggest that local brain hypothermia might require additional therapeutic protocols to be applied in combination.
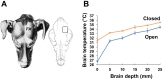
Regarding this, in our 2013 study, we developed a prolonged and delayed local brain hypothermia protocol in the tMCAO rat model [10]. For that study, we made a more compact cooling coil than the one previously developed by Clark and Colbourne [16]. We initiated delayed local hypothermia 4 hours after reperfusion onset. The target brain temperature (31℃) was obtained 25 minutes after the initiation of cooling and was maintained for the remainder of the experiment (44 hours). The brain temperature in the ipsilateral cortex (depth 3 mm) was ~31℃, whereas the temperature in the ipsilateral striatal area (depth 3 mm) and in the contralateral cortex (depth 3 mm) was ~34℃. Prolonged local hypothermia did not induce systemic hypothermia. After application, brain infarct area was significantly decreased from 68% of the hemisphere in the normothermia group to 26% of the hemisphere in the hypothermia group, and the loss of neurovascular molecules was prevented by local hypothermia. Yet, we postulated that the efficiency of local hypothermia would likely be further increased by small organic thermoconduction. Therefore, we executed hemispheric local hypothermia using a similar experimental procedure in the canine model (Fig. 1). Additionally, local hypothermia was conducted after decompressive craniectomy due to clinical practicalities. Decompressive craniectomy is a neurosurgical procedure in which part of the skull is removed to reduce intracranial pressure (ICP), thereby making room for a swollen brain to expand without being squeezed. Decompressive craniectomy can be used as a window to provide local hypothermic treatment to the brain without the systemic complications [19, 20]. A hemicraniectomy was created by removing a bone flap (approximately 5 cm×3 cm). Brain cooling was induced by irrigation with cold saline (~20℃) and temperature was monitored with a thermometer placed on the surface of the brain (Fig. 1). Due to exposure of the brain to the cold air in the surgery room and to anesthesia, the baseline brain temperature (~35℃) was lower than the body core temperature (~37℃). Nevertheless, the brain temperature gradient had a rate of 1℃ per 5 mm of depth in both the parietal cortex (the opened and cooling region) and the temporal cortex (the closed and non-cooling region). A favorable reduction in brain temperature (to 33℃) was also obtained in the deep brain. No physiological or histological abnormalities were observed. Next, to evaluate the neuroprotective effects of local hypothermia, we applied our cooling system and polyethylene pad (approximately 5 cm×5 cm) to a canine brain ICH model induced by autologous blood injection (Fig. 2). The cooling pad was implanted at the site of the craniectomy and brain cooling was initiated 4 hours after ICH and maintained for 12 hours. The brain was then rewarmed until magnetic resonance (MR) image acquisition was completed, after which the animal was sacrificed. Images were acquired at 0.3 Tesla with T2-weighted spin-echo sequences obtained in the coronal plane. MR images showed that ICH-induced brain edema was successfully prevented in the hypothermic area (~0.4% v/total v, below 33℃) even though hypothermic treatment was delayed (Fig. 3A).
After brain injury, edema began increasing at 1-hour post-injury, peaked at 24 hours post-injury, and remained elevated for 4 days. The therapeutic window for hypothermia has been reported as administration within 60~90 minutes after injury in the rat ischemic and TBI models [21, 22]. On the contrary, when we delayed hypothermic treatment by more than 90 minutes, we found that the delay did not affect the pattern of neuropathology in our study. Our local hypothermia protocol made possible significant beneficial outcomes, even when hypothermic initiation was delayed 4 hours after injury. Craniectomy combined with local hypothermia probably played a role in making a new therapeutic window as well as in overcoming thermo-barriers such as the skin and skull, which is one of the limitations of local brain hypothermia.
CELLULAR MECHANISMS OF NEUROPROTECTIVE THERAPEUTIC HYPOTHERMIA
Therapeutic hypothermia can induce a wide range of neuroprotective molecular mechanisms including modulating excitatory amino acid release and free radical formation, enhancing small ubiquitin-related pathways and protein kinase C activity, and slowing cellular metabolism [23, 24, 25, 26]. The cerebral metabolic rate decreases by 6% for every 1℃ reduction in brain temperature [26]. Therapeutic hypothermia decreases the loss of high-energy organic phosphates, slows the rates of metabolite consumption and lactic acid accumulation, and reduces cerebral metabolic oxygen consumption while improving glucose utilization [27].
We investigated the modulation of glia by local hypothermia
CONCLUSION AND FUTURE PROSPECTS
The aims of this review have been to provide a comprehensive picture of the practicality of using local brain hypothermia to treat brain injury, and to discuss the cellular mechanisms-in particular the modulation of glia-by which local hypothermia leads to neuroprotection. Local hypothermia is a promising therapeutic intervention after TBI and stroke and has advantages over systemic hypothermia. However, most local cooling interventions remain in their experimental stages due to the lack of evidence regarding their efficacy, to the presence of unwanted side effects, to their unproven practicality and feasibility, and to their unknown mechanisms of action. The best strategy of hypothermic treatment may require the combination of all advantages of systemic hypothermia, without the limitation of local hypothermia (Fig. 4). Toward this end, we have tried delayed and prolonged local brain hypothermia combined with decompressive craniectomy, which lowers ICP in patients with a high risk of mortality. The combination of hypothermic treatment and craniectomy led to positive outcomes in our animal models; therefore, it is a promising intervention strategy to increase the survival rate of patients with brain injury by modulating the activation of their glial cells and thereby preventing secondary injury.
Figures
References
- Safar P. Community-Wide Cardiopulmonary Resuscitation. J Iowa Med Soc 1964;54:629-635.
- Mareedu RK, Grandhe NP, Gangineni S, Quinn DL. Classic EKG changes of hypothermia. Clin Med Res 2008;6:107-108.
- Bassin L, Yong AC, Kilpatrick D, Hunyor SN. Arrhythmogenicity of hypothermia - a large animal model of hypothermia. Heart Lung Circ 2014;23:82-87.
- Geurts M, Macleod MR, Kollmar R, Kremer PH, van der Worp HB. Therapeutic hypothermia and the risk of infection: a systematic review and meta-analysis. Crit Care Med 2014;42:231-242.
- Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med 1992;20:1402-1405.
- Perman SM, Goyal M, Neumar RW, Topjian AA, Gaieski DF. Clinical applications of targeted temperature management. Chest 2014;145:386-393.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009;37:S186-S202.
- Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol 2002;17:1137-1152.
- Seo JW, Kim JH, Seo M, Han HS, Park J, Suk K. Time-dependent effects of hypothermia on microglial activation and migration. J Neuroinflammation 2012;9:164.
- Kim JH, Seo M, Han HS, Park J, Suk K. The neurovascular protection afforded by delayed local hypothermia after transient middle cerebral artery occlusion. Curr Neurovasc Res 2013;10:134-143.
- Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honings D, Rodde M, Burnham J, Wang D. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg 2004;100:272-277.
- Liu WG, Qiu WS, Zhang Y, Wang WM, Lu F, Yang XF. Effects of selective brain cooling in patients with severe traumatic brain injury: a preliminary study. J Int Med Res 2006;34:58-64.
- Qiu W, Shen H, Zhang Y, Wang W, Liu W, Jiang Q, Luo M, Manou M. Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci 2006;13:995-1000.
- Abou-Chebl A, Sung G, Barbut D, Torbey M. Local brain temperature reduction through intranasal cooling with the RhinoChill device: preliminary safety data in brain-injured patients. Stroke 2011;42:2164-2169.
- Covaciu L, Allers M, Lunderquist A, Rubertsson S. Intranasal cooling with or without intravenous cold fluids during and after cardiac arrest in pigs. Acta Anaesthesiol Scand 2010;54:494-501.
- Clark DL, Colbourne F. A simple method to induce focal brain hypothermia in rats. J Cereb Blood Flow Metab 2007;27:115-122.
- Clark DL, Penner M, Wowk S, Orellana-Jordan I, Colbourne F. Treatments (12 and 48 h) with systemic and brain-selective hypothermia techniques after permanent focal cerebral ischemia in rat. Exp Neurol 2009;220:391-399.
- Fingas M, Penner M, Silasi G, Colbourne F. Treatment of intracerebral hemorrhage in rats with 12 h, 3 days and 6 days of selective brain hypothermia. Exp Neurol 2009;219:156-162.
- Hofmeijer J, Schepers J, Veldhuis WB, Nicolay K, Kappelle LJ, Bar PR, van der Worp HB. Delayed decompressive surgery increases apparent diffusion coefficient and improves peri-infarct perfusion in rats with space-occupying cerebral infarction. Stroke 2004;35:1476-1481.
- Doerfler A, Engelhorn T, Forsting M. Decompressive craniectomy for early therapy and secondary prevention of cerebral infarction. Stroke 2001;32:813-815.
- Zhao H, Steinberg G. Limited Therapeutic Time Windows of Mild-to-Moderate Hypothermia in a Focal Ischemia Model in Rat. Stroke Res Treat 2011;2011:131834.
- Markgraf CG, Clifton GL, Moody MR. Treatment window for hypothermia in brain injury. J Neurosurg 2001;95:979-983.
- Yenari MA, Colbourne F, Hemmen TM, Han HS, Krieger D. Therapeutic hypothermia in stroke. Stroke Res Treat 2011;2011:157969.
- Liu L, Yenari MA. Therapeutic hypothermia: neuroprotective mechanisms. Front Biosci 2007;12:816-825.
- Berger C, Schabitz WR, Georgiadis D, Steiner T, Aschoff A, Schwab S. Effects of hypothermia on excitatory amino acids and metabolism in stroke patients: a microdialysis study. Stroke 2002;33:519-524.
- Steen PA, Newberg L, Milde JH, Michenfelder JD. Hypothermia and barbiturates: individual and combined effects on canine cerebral oxygen consumption. Anesthesiology 1983;58:527-532.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab 2003;23:513-530.
- Kim JH, Seo M, Suk K. Effects of therapeutic hypothermia on the glial proteome and phenotype. Curr Protein Pept Sci 2013;14:51-60.
- Seo M, Kim JH, Cho YE, Baek MC, Suk K. Hypothermic regulation of astrocyte proteome profile in experimental stroke. Electrophoresis 2012;33:3835-3848.
- Kim JH, Cho YE, Seo M, Baek MC, Suk K. Glial proteome changes in response to moderate hypothermia. Proteomics 2012;12:2571-2583.
- Diestel A, Troeller S, Billecke N, Sauer IM, Berger F, Schmitt KR. Mechanisms of hypothermia-induced cell protection mediated by microglial cells in vitro. Eur J Neurosci 2010;31:779-787.
- Matsui T, Kakeda T. IL-10 production is reduced by hypothermia but augmented by hyperthermia in rat microglia. J Neurotrauma 2008;25:709-715.
- Sussman BJ, Barber JB, Goald H. Experimental intracerebral hematoma. Reduction of oxygen tension in brain and cerebrospinal fluid. J Neurosurg 1974;41:177-186.
- Sukstanskii AL, Yablonskiy DA. Theoretical limits on brain cooling by external head cooling devices. Eur J Appl Physiol 2007;101:41-49.
- Choi JH, Marshall RS, Neimark MA, Konstas AA, Lin E, Chiang YT, Mast H, Rundek T, Mohr JP, Pile-Spellman J. Selective brain cooling with endovascular intracarotid infusion of cold saline: a pilot feasibility study. AJNR Am J Neuroradiol 2010;31:928-934.