Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2022; 31(4): 260-269
Published online August 31, 2022
https://doi.org/10.5607/en22010
© The Korean Society for Brain and Neural Sciences
Anti-stress Effect of Octopus Cephalotocin in Rats
Ye-Ji Kim1,2, Seonmi Jo3, Seung-Hyun Jung3 and Dong Ho Woo1,2*
1Research Center for Convergence Toxicology, Korea Institute of Toxicology, Daejeon 34114,
2Department of Human and Environmental Toxicology, University of Science and Technology, Daejeon 34114,
3Department of Genetic Resources, National Marine Biodiversity Institute of Korea, Seocheon 33662, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-42-610-8243, FAX: 82-42-610-8252
e-mail: dongho.woo@kitox.re.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
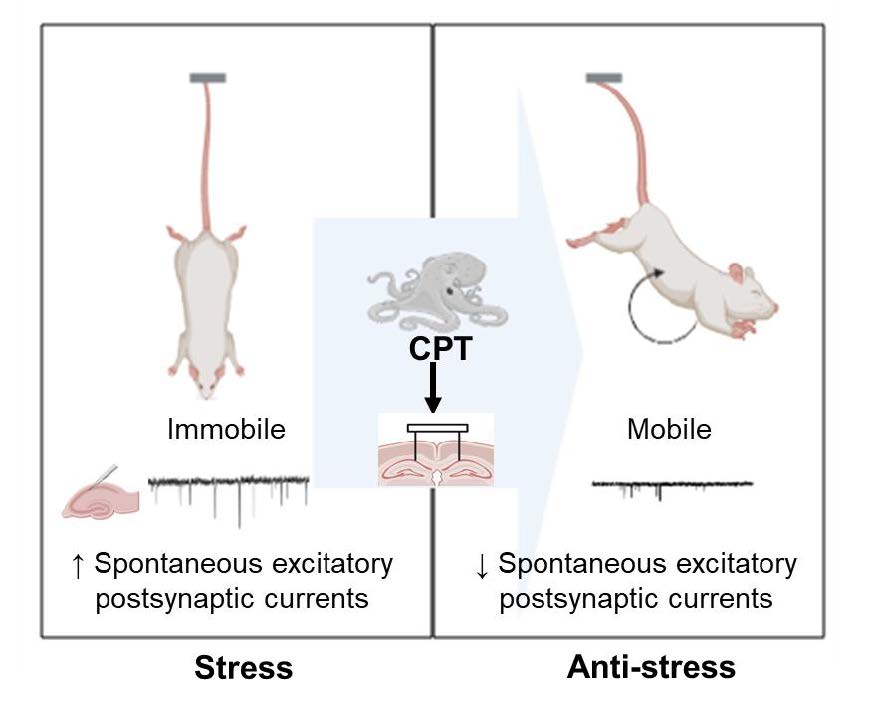
Cephalotocin is a bioactivity-regulating peptide expressed in octopus (
Graphical Abstract
Keywords: Cephalotocin, SD rat, Tail suspension test, sEPSCs, Intrahippocampal infusion


