Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2022; 31(4): 260-269
Published online August 31, 2022
https://doi.org/10.5607/en22010
© The Korean Society for Brain and Neural Sciences
Anti-stress Effect of Octopus Cephalotocin in Rats
Ye-Ji Kim1,2, Seonmi Jo3, Seung-Hyun Jung3 and Dong Ho Woo1,2*
1Research Center for Convergence Toxicology, Korea Institute of Toxicology, Daejeon 34114,
2Department of Human and Environmental Toxicology, University of Science and Technology, Daejeon 34114,
3Department of Genetic Resources, National Marine Biodiversity Institute of Korea, Seocheon 33662, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-42-610-8243, FAX: 82-42-610-8252
e-mail: dongho.woo@kitox.re.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Cephalotocin is a bioactivity-regulating peptide expressed in octopus (
Graphical Abstract
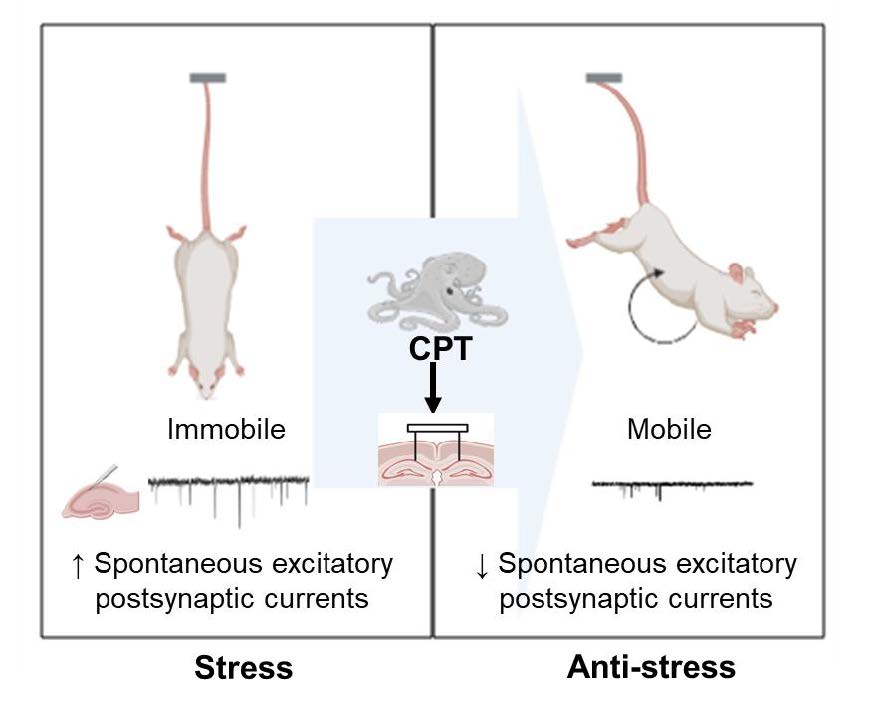
Keywords: Cephalotocin, SD rat, Tail suspension test, sEPSCs, Intrahippocampal infusion
INTRODUCTION
Arginine vasopressin (Avp) is an important regulator of stress. Vasopressin-deficient Brattleboro rats have been shown to exhibit lower stress levels than wild-type rats in a 5 min forced swim test but not after a 15 min test, suggesting that Avp is strongly correlated with stress regulation [1]. The Avp 1a receptor (Avpr1a) is related to vascular modulation [2, 3], the Avp 2 receptor (Avpr2) is related to renal duct water reabsorption [4], and the Avp 1b receptor (Avpr1b or V3R) is related to stress regulation. Avpr1b plays key roles in social memory, aggressive behavior and stress responses [5]. An acute 38℃ treatment increases the blood Avp levels and decreases Avpr1b protein expression, suggesting that Avpr1b is strongly related to stress regulation [6].
The sequence of an octopus-originated peptide, cephalotocin (CPT), is similar to that of Avp and oxytocin. CPT has the potential to interact with the human oxytocin receptor and the vasopressin receptor. The 397 amino acid sequence of the receptor of CPT shares close homology with the receptor of vasopressin and is a potential Gq protein-coupled receptor that activates Ca2+-activated Cl- currents in the
Vasopressin treatment is not only involved in stress control by regulating hippocampal Avp1b levels [5, 7, 11] but also related to the physiological process of diuretic reabsorption [4]. As a result, we propose a link between brain stress and diuretic reabsorption. This study is the first to assess the effect of the peptide CPT generated from octopus genome information on stress regulation.
The purpose of the present study is to show for the first time the results and the process for determining whether CPT is effective in relieving stress in mammals. CPT decreases the frequency of glutamate release recorded from cultured rat hippocampal neurons and mouse hippocampal CA1 pyramidal neurons. Intraperitoneal injection does not change locomotion in the open field test (OFT), and intrahippocampal infusion significantly reduces the immobility time in the tail suspension test (TST).
MATERIALS AND METHODS
Peptide
As described in Table 1, high purity of CPT were synthesized by AnyGen (Gwangju, Korea) using solid-phase peptide synthesis. The purity and molecular masses of the peptides were determined using high-performance liquid chromatography (HPLC) and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) (Shimadzu, Kyoto, Japan).
Preparation of primary cultures of rat hippocampal neurons
Primary cultures of hippocampal neurons were prepared from postnatal day (P) 0-P1 rats using a previously described method to record EPSCs [12, 13]. The hippocampus was dissected and incubated with Mg2+- and Ca2+-free HBSS (Gibco, Massachusetts). The dissected brains were trypsinized with 0.025% trypsin for 10 min at 37℃. Trypsin activity was stopped by adding DMEM containing 10% FBS and incubating the samples for 1 min at room temperature. The medium was decanted and replaced with neurobasal medium (Gibco, Massachusetts) containing 2% B27 supplement, 2 mM L-glutamine, and 1% penicillin–streptomycin (100 units/ml penicillin and 100 μg/ml streptomycin, Gibco). The cell suspension was passed through a fire-polished Pasteur pipette and incubated for 2 min. The trituration process was repeated 7-10 times. The cells were passed through a cell strainer with a pore size of 100 µm (93100, SPL, Seoul, Korea) and then filtered through another cell strainer with a pore size of 40 µm (93040, SPL, Korea) to remove nontriturated tissue debris. The cells were plated at a density of 3×105 cells per cover glass. The cultures were maintained in a humidified atmosphere containing 5% CO2 and 95% O2 at 37℃ for 14~20 days.
Preparation of mouse hippocampal slices
Subsequently, we performed experiments to record the action potentials (APs) and excitatory postsynaptic currents (EPSCs) in mouse hippocampal pyramidal neurons. Four-week-old C57BL/6 male mice were anesthetized with 20 mg/ml avertin (2,2,2-tribromoethanol, Sigma–Aldrich, MA, USA; cat. no. #T4, 840-2). The brain was quickly removed from each mouse and horizontally sectioned in artificial cerebrospinal fluid (ACSF) consisting of (in mM) 130 NaCl, 1.25 NaH2PO4, 3.5 KCl, 24 NaHCO3, 1.5 CaCl2, 1.5 MgCl2 and 10 glucose. Rostral-to-caudal 300-μm-thick brain slices containing the hippocampal region were cut using a vibratome (Leica VT1000 S, Wetzlar, Germany) and bubbled with 95% O2/5% CO2 at room temperature.
Electrophysiology
Whole-cell patch-clamp recordings of cultured hippocampal neurons and mouse hippocampal slices under voltage clamp (holding potential -70 mV) were performed with a MultiClamp 700B amplifier and digitized with a Digidata 1322A data acquisition system (Molecular Devices, CA, USA). Borosilicate glass capillaries (cat. no. 1B150F-4, outer diameter, 1.50 mm; inner diameter, 1 mm; World Precision Instruments, FL, USA) with a tip resistance of 3~8 MΩ were pulled (P-97 micropipette puller, Sutter Instrument, Novato, CA, USA). The capillaries were filled with an intracellular solution composed of (in mM) 150 CsMeSO4, 10 NaCl, 0.5 CaCl2, and 10 HEPES (pH adjusted to 7.3 with CsOH and osmolarity adjusted to 310 mOsm/L). The extracellular solution used for the cell patch-clamp recording was composed of (in mM) 150 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2 10 glucose and 10 HEPES (pH adjusted to 7.4 with NaOH and osmolarity adjusted to 315~320 mOsm/L with sucrose), and the ACSF solution used for the slice patch-clamp recording was composed of (in mM) 130 NaCl, 1.25 NaH2PO4, 3.5 KCl, 24 NaHCO3, 1.5 CaCl2, 1.5 MgCl2 and 10 glucose (osmolarity adjusted to 330~340 mOsm/L). QX 314 (0.5 mM) was used to inhibit voltage-gated sodium channels. Mini Analysis software (Synaptosoft, USA) was used to analyze the frequency and amplitude.
AP recordings of mouse hippocampal neurons were performed in the current clamp mode with a MultiClamp 700B amplifier and digitized with a Digidata 1322A data acquisition system (Molecular Devices, CA, USA). The glass capillaries were filled with an intracellular solution consisting of (in mM) 140 K-gluconate, 10 HEPES, 7 NaCl, 4 Mg-ATP, and 0.3 Na-GTP (pH adjusted to 7.4 with KOH and osmolarity adjusted to 280~290 mOsm/L). Each patched cell was inspected in the current clamp mode, and neurons were subjected to 10 depolarizing current pulses in 20 pA increments for 0.5 s each at 2 s intervals; then, the membrane potentials, which were initially adjusted to -60 mV, were returned to the resting potential.
Immunohistochemistry (IHC) of rat brain tissue sections
Rats were anesthetized with 20 mg/ml avertin and perfused with 0.9% saline, followed by ice-cold 4% paraformaldehyde (PFA). The brains were postfixed in PFA overnight and 30% sucrose for 48 hrs at 4℃. Coronal sections were cut with a cryostat at 20 µm and attached to glass slides. The sections were washed with PBS and incubated for 1 hr with a blocking solution (0.5% Triton X-100, 0.5% BSA, and 5% normal goat serum in PBS). The sections were incubated with primary antibodies in blocking solution overnight on a shaker at 4℃. After washing with 0.5% Triton X-100 in PBS (PBST) twice and 0.5% Triton X-100 and 0.5% BSA in PBS (PBSTB) once for 10 min at room temperature, the sections were incubated with secondary antibodies in PBSTB for 1 hr at room temperature. After three washes with PBST and 1 wash with PBS for 10 min at room temperature, the sections were mounted with mounting medium containing DAPI (VECTASHIELD, CA, USA) and dried. A series of fluorescence images were obtained under a confocal microscope (Olympus, Tokyo, Japan) and analyzed using ImageJ software. The primary antibody, anti-Avpr1b (AVR-011, Alomone, Jerusalem, Israel), was diluted 1:200, and the secondary antibody, Alexa Fluor 594-conjugated goat anti-rabbit (A11012, Invitrogen, MA, USA), was diluted 1:1,000.
Intraperitoneal injection (IP) and open field test (OFT)
Four-week-old C57BL/6 male mice were first acclimated for one week after acquisition before assessing their activity. The mice were grouped based on body weight. The mice were acclimated in polycarbonate cages with bedding. The mice were group-housed under a 12-h light/dark cycle at 25℃ with free access to food and water. The mice were fasted for 12 hours to maximize the effect of the peptide on behavioral activity. First, 1 mg/ml or 5 mg/ml CPT or ethanol (EtOH, positive control) was intraperitoneally injected by a trained experimenter using a 26-gauge thick syringe after weighing the animal. The box used for the OFT was equipped with a recording camera. Each mouse was placed in the center of the box, habituated for 30 min and then tested for 1 hr after an intraperitoneal injection of 1 mg/ml/kg CPT with a 1 ml syringe. The tests were videotaped, and the distances traveled by the mice were analyzed using EthoVisionXT software (version 14, Noldus, Netherlands).
Stereotaxic surgery, CPT injection and the tail suspension test (TST) in rats
Four-week-old male Sprague–Dawley (SD) rats were obtained, and five-week-old rats were subjected to stereotactic surgery and injected with CPT in the CA1 region of the hippocampus before the TST. The SD rats were single-housed under a 12-h light/dark cycle at 25℃ with free access to food and water. The rats were anesthetized with 2% isoflurane and placed in a stereotactic frame with sustained anesthesia during surgery. The scalp was incised from the frontal cranial bones to the back using a scalpel blade. Holes were drilled into the skull above the hippocampus (anterior/posterior, -2.0 mm; medial/lateral, -1.5 or +1.5 mm from the bregma) for the placement of the injection needle. An intracerebral guide cannula was inserted into the hippocampal CA1 region (dorsal/ventral, -1.7 mm), and a dummy cannula was inserted into the guide cannula to prevent blockage of the holes. After 5 days of recovery, 1 mg/2 µl of CPT dissolved in saline solution was loaded and injected for 2 min into six-week-old SD rats to measure stress behaviors.
TST
We used rats that were stereotaxically injected in the hippocampal CA1 region to measure stress. After the injection, the rats were suspended from the tail above the floor with adhesive tape using a traction force meter. The rats were placed in the middle of the horizontal plane, and the tests lasted for 10 min. The tests were videotaped and scored by a trained observer.
All animal protocols were performed in strict accordance with the institutional Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Korea Institute of Toxicology (1901-0028).
Data analysis
The experimental data are presented as the means±the experimental standard errors of the means (error bars), which were calculated using Excel 2016 software (Microsoft, WA, USA). The EC50 (concentration producing a half-maximal response) and IC50 (concentration causing half-maximal inhibition of the control agonist response) values were determined by 4-parameter logistic fitting of the dose–response curve using SigmaPlot 10.0 (Systat Software, CA, USA). The statistical analyses were performed using GraphPad Prism version 8. The comparisons were performed using a paired t test, an unpaired t test or one-way ANOVA. Nonsignificant: ns, p>0.05; significant: *p<0.05, **p<0.01, and ***p<0.001, as indicated in the individual figures.
RESULTS
CPT decreases both the frequency and amplitude of spontaneous EPSCs (sEPSCs) recorded from both cultured pyramidal neurons of SD rats and CA1 pyramidal neurons in C57BL/6 mouse brain slices
Avp modulates social memory via hippocampal Avpr1b-dependent changes [14], increases GABA release and reduces the excitability of hippocampal activity [15]. Avp increases the excitability of SD rat hippocampal neurons by increasing APs [10]. We investigated whether CPT, an Avp analog whose amino acid sequence differs from that of Avp at only the 4th and 8th residues, changes excitability in rat hippocampal neurons (Fig. 1a). CPT (10 µM) decreased the frequency of sEPSCs (Fig. 1b, c, right shifted red line in d, paired t test, *p=0.04). CPT also decreased the amplitude of sEPSCs (Fig. 1b, e, left-shifted red line in d and f, paired t test, *p=0.04). These results are inconsistent with a previous finding [10] suggesting that vasopressin substantially increases the number of APs. Then, CPT was applied to mouse hippocampal CA1 pyramidal neurons in the current clamp mode, and the number of APs was recorded (Fig. 2a, b). The number of APs increased in the presence of 10 µM CPT and a 20-pA current (Fig. 2c, e, paired t test, *p=0.03). At least in terms of the quantity of APs, vasopressin and CPT produced different patterns in neuronal excitability. CPT was applied to mouse hippocampal neurons (Fig. 3a, b), and the sEPSC frequency in the CPT group was significantly lower than that in the non-CPT group (Fig. 3c, d, right-shifted red line in e, paired t test, *p=0.03). In addition, CPT decreased the amplitude of sEPSCs (Fig. 3c, f, left-shifted red line in g, paired t test, *p=0.04).
IP injection of 1 mg/kg CPT does not change locomotor activity
The effects of CPT on the behavior of mammals have not been reported. In the present study, the OFT was used to determine the effect of CPT on locomotor activity (Fig. 4a). An IP injection of 1 mg/kg CPT did not change locomotor activity according to the measurement of the distance traveled in the OFT (Fig. 4b, d, e) in contrast to an injection of ethanol, which significantly increased the distance traveled (Fig. 4b, c, e, one-way ANOVA, *p=0.02 saline and ethanol).
An intrahippocampal infusion of CPT significantly reduces the immobility time in the TST
We directly administered CPT into the brain because the intraperitoneal injection of CPT did not change locomotion, thus indicating that a systemic injection of CPT does not alter general locomotion. Thus, the reduced immobility following the intrahippocampal injection of CPT is not due to changes in motor function. Avpr1b (or V3R) is related to stress regulation. The expression of the Avpr1b transcript has been detected in many regions of the rodent nervous system, including the medulla, cerebellum, pons, midbrain, caudate putamen, septum, hypothalamus, piriform cortex, amygdala, and parietal cortex. However, no expression has been observed in the vomeronasal organ, olfactory bulb, or thalamus [7]. In the current study, Avpr1b was mainly expressed in the hippocampal CA1 region (Fig. 5a~o). The number of Avpr1b-expressing cells was divided by the number of DAPI-expressing cells to determine the average number of Avpr1b-expressing cells (Fig. 5p~r). The intrahippocampal infusion was performed through an implanted cannula (Fig. 6a, b). We monitored the durations of mobility and immobility (Fig. 6c) during the indicated procedures (Fig. 6d). The mobility duration was increased in the presence of CPT (Fig. 6e, unpaired t test, **p=0.01 saline & CPT). The immobility duration was significantly decreased in the presence of CPT (Fig. 6f, unpaired t test, **p=0.01 saline & CPT).
DISCUSSION
Based on our results, an intrahippocampal infusion of CPT increases the mobile time in the TST through the molecular mechanism of reduced glutamate release in hippocampal pyramidal CA1 neurons.
The goal of this research was to determine whether CPT derived from octopus genomic data is bioavailable to mammals. The results of this study suggest that the peptide CPT synthesized according to previous octopus studies ameliorates stress by inhibiting glutamate release from hippocampal pyramidal neurons. Hippocampal stimulation with 1 s current trains of 1 s on /1 s off (100 mA, 50~60 Hz, 1 ms pulse duration) for 10 min has been shown to increase the plasma corticosterone levels by 20% beginning at 5 min and continuing for 30 min [16]. In addition, monophasic stimulation with a 1.3 V current for 0.1 ms has been reported to reduce blood corticosterone levels [17]. These findings suggest that hippocampal electrical stimulation in vivo modulates the hippocampal–pituitary–adrenal (HPA) axis. In this paper, an acute treatment with CPT reduced the stress levels by inhibiting presynaptic glutamate release and increasing CA1 APs.
The ventral region of the hippocampus is related to emotions, whereas the dorsal region is responsible for spatial memory [18]. We observed a discrepancy because CPT injection in the dorsal hippocampal region relieved stress, which is known to be mediated by the ventral hippocampal region. Nevertheless, extirpation of the dorsal hippocampus increased the blood levels of beta-endorphins, which is related to reduced stress [19]. The hippocampus performs a specific function in stress regulation in addition to memory and learning [20]. In addition, because the activation of Avpr1b regulates the release of stress hormones to relieve stress [21, 22] and the expression of Avpr1b in the hippocampus [7], the activity of Avpr1b in the hippocampus might regulate stress. We propose that dorsal hippocampal Avpr1b is involved in a specific mechanism that regulates stress.
The binding of plasma corticosterone secreted from the kidney adrenal cortex to the gonadotropin receptor (GR) in the hippocampus reduces stress levels through negative feedback [23]. Corticotropin–releasing hormone (CRH) neurons located in the paraventricular nucleus (PVN) release CRH and regulate ACTH release from the pituitary. ACTH activates the release of corticosterone from the adrenal cortex. Subsequently, the increased corticotropin levels bind GR, which, in turn, functions as a transcription factor to repress the pathway and reduce stress levels [24]. Avp may act via negative feedback to reduce stress levels by facilitating the GR pathway. The reduced glutamate release in the hippocampal CA3 regulates the stress axis, namely, the hypothalamus-pituitary gland-adrenal cortex axis. The reduced glutamate release in the hippocampus may regulate hormone release from the PVN and pituitary gland. Glutamate release measured from hippocampal CA1 pyramidal neurons was also reduced. Consistently, our results are similar to previous findings.
Avp significantly increased the numbers of APs recorded from hippocampal CA1 pyramidal neurons from 20- to 30-day-old SD rats. An intracellular infusion of GDP-β-S, a nonhydrolyzable G protein, and U73122, a phospholipase C (PLC) inhibitor, does not increase Avp-induced excitability, suggesting that Avp-induced excitability requires PLCβ [10]. In the current study, CPT decreased both the frequency and amplitude of sEPSCs in hippocampal neurons from SD rats. Avp substantially increased the number of APs by inhibiting the inward rectifying potassium channel (GIRK, [10]), but CPT slightly increased the number of APs under only one condition (Fig. 2), suggesting that the affinity of CPT to Avp receptors differs from that of Avp. A possible explanation is the multireceptor activation of different subtypes of Avpr by CPT. For example, the interaction between CPT and Avpr1b is quite different in dissociating G proteins to regulate the mechanism of AP firing. High Avp1b expression is detected in the CA2 region of the dorsal hippocampus, while it is expressed at equal levels in the dorsal and ventral CA3, CA2 and CA1. Avpr1b expression has also been detected in dense fibers and numerous glial cells in the stratum oriens and stratum radiatum [25], suggesting that Avpr1b is expressed in the presynaptic membrane, postsynaptic membrane and glial membrane. Even if CPT possesses anti-stress properties, more research is needed to determine whether this peptide migrates into the brain. This study is important for the development of CPT as an anti-stress agent.
An intrahippocampal infusion of CPT, an Avp analog, exerted an anti-stress effect. The mechanism involved a reduction in glutamate release from the presynaptic terminals of hippocampal neurons. The CA1 CPT treatment mimicked Avp release from CRH neurons of the PVN in the presence of increasing stress levels during the TST. The primary significance of this work are that a peptide generated from octopus-derived genetic information exerts anti-stress effects in mammals and that the mechanism involves blockade of glutamate release in hippocampal neurons.
ACKNOWLEDGEMENTS
We thank Mr. Choi Chang-Hoon (KIT), who supported the cephalotocin injections and animal care, and Dr. Hye Suck An (MABIK) for obtaining funding and organizing stimulating research.
COMPETING FINANCIAL INTERESTS
The South Korean government certified the patent (South Korea Patent 10-2239947) titled “Compositions for preventing, improving or treating cognition and stress-related disease comprising cephalotocin” on April 08, 2021, as a beneficiary. YJ Kim and DH Woo are listed as inventors in the patent application.
FUNDING
This work was supported by grants from the Collaborative Genome Program of the Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (MOF) (No. 20180430 to DHW), grant 20182MFDS423 from the Ministry of Food and Drug Safety in 2022, the Republic of Korea (to DHW), a grant from the Korea Institute of Toxicology (KIT) Research Program (1711159828) and a grant from the National Marine Biodiversity Institute (MABIK) Research Program (No. 2022M00400 to SJ) funded by the Ministry of Oceans and Fisheries, Korea.
AUTHOR CONTRIBUTIONS
YJK performed the in vitro and ex vivo electrophysiological experiments and rodent behavioral experiments. SJ and SHJ developed the initial concept and contributed to the experimental design based on a previous study investigating CPT (not yet published). DHW conceived the study design and wrote the manuscript.
Figures






Tables
References
- Zelena D, Pintér O, Balázsfi DG, Langnaese K, Richter K, Landgraf R, Makara GB, Engelmann M (2015) Vasopressin signaling at brain level controls stress hormone release: the vasopressin-deficient Brattleboro rat as a model. Amino Acids 47:2245-2253
- Morel A, O'Carroll AM, Brownstein MJ, Lolait SJ (1992) Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature 356:523-526
- Koshimizu TA, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, Adachi T, Tanaka T, Kuwaki T, Mori T, Takeo S, Okamura H, Tsujimoto G (2006) V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci U S A 103:7807-7812
- Knepper MA (1997) Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol 272(1 Pt 2):F3-F12
- Caldwell HK, Aulino EA, Rodriguez KM, Witchey SK, Yaw AM (2017) Social context, stress, neuropsychiatric disorders, and the vasopressin 1b receptor. Front Neurosci 11:567
- Jasnic N, Djordjevic J, Vujovic P, Lakic I, Djurasevic S, Cvijic G (2013) The effect of vasopressin 1b receptor (V1bR) blockade on HPA axis activity in rats exposed to acute heat stress. J Exp Biol 216(Pt 12):2302-2307
- Young WS, Li J, Wersinger SR, Palkovits M (2006) The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 143:1031-1039
- Kanda A, Takuwa-Kuroda K, Iwakoshi-Ukena E, Furukawa Y, Matsushima O, Minakata H (2003) Cloning of octopus cephalotocin receptor, a member of the oxytocin/vasopressin superfamily. J Endocrinol 179:281-291
- Reich G (1992) A new peptide of the oxytocin/vasopressin family isolated from nerves of the cephalopod Octopus vulgaris. Neurosci Lett 134:191-194
- Hu B, Boyle CA, Lei S (2022) Roles of PLCβ, PIP2, and GIRK channels in arginine vasopressin-elicited excitation of CA1 pyramidal neurons. J Cell Physiol 237:660-674
- Stevenson EL, Caldwell HK (2012) The vasopressin 1b receptor and the neural regulation of social behavior. Horm Behav 61:277-282
- Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY, Oh SJ, Hwang EM, Marmorstein AD, Bae YC, Park JY, Lee CJ (2012) TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151:25-40
- Bahrami F, Bahari Z, Abolghasemi R, Golmanesh L, Meftahi GH (2020) The neuroprotective effects of stimulation of NMDA receptors against POX-induced neurotoxicity in hippocampal cultured neurons; a morphometric study. Mol Cell Toxicol 16:401-408
- Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, Young WS (2016) Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol Psychiatry 21:1137-1144
- Spoljaric A, Seja P, Spoljaric I, Virtanen MA, Lindfors J, Uvarov P, Summanen M, Crow AK, Hsueh B, Puskarjov M, Ruusuvuori E, Voipio J, Deisseroth K, Kaila K (2017) Vasopressin excites interneurons to suppress hippocampal network activity across a broad span of brain maturity at birth. Proc Natl Acad Sci U S A 114:E10819-E10828
- Dunn JD, Orr SE (1984) Differential plasma corticosterone responses to hippocampal stimulation. Exp Brain Res 54:1-6
- Dupont A, Bastarache E, Endröczi E, Fortier C (1972) Effect of hippocampal stimulation on the plasma thyrotropin (THS) and corticosterone responses to acute cold exposure in the rat. Can J Physiol Pharmacol 50:364-367
- Moser MB, Moser EI (1998) Functional differentiation in the hippocampus. Hippocampus 8:608-619
- Herman JP, Schäfer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ (1989) Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci 9:3072-3082
- Buchanan TW, Tranel D, Kirschbaum C (2009) Hippocampal damage abolishes the cortisol response to psychosocial stress in humans. Horm Behav 56:44-50
- Lolait SJ, O'Carroll AM, Mahan LC, Felder CC, Button DC, Young WS 3rd, Mezey E, Brownstein MJ (1995) Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci U S A 92:6783-6787
- Saito M, Sugimoto T, Tahara A, Kawashima H (1995) Molecular cloning and characterization of rat V1b vasopressin receptor: evidence for its expression in extra-pituitary tissues. Biochem Biophys Res Commun 212:751-757
- Jacobson L, Sapolsky R (1991) The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12:118-134
- Kim JS, Iremonger KJ (2019) Temporally tuned corticosteroid feedback regulation of the stress axis. Trends Endocrinol Metab 30:783-792
- Williams Avram SK, Lee HJ, Fastman J, Cymerblit-Sabba A, Smith A, Vincent M, Song J, Granovetter MC, Lee SH, Cilz NI, Stackmann M, Chaturvedi R, Young WS (2019) NMDA receptor in vasopressin 1b neurons is not required for short-term social memory, object memory or aggression. Front Behav Neurosci 13:218





