Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2018; 27(1): 45-56
Published online February 28, 2018
https://doi.org/10.5607/en.2018.27.1.45
© The Korean Society for Brain and Neural Sciences
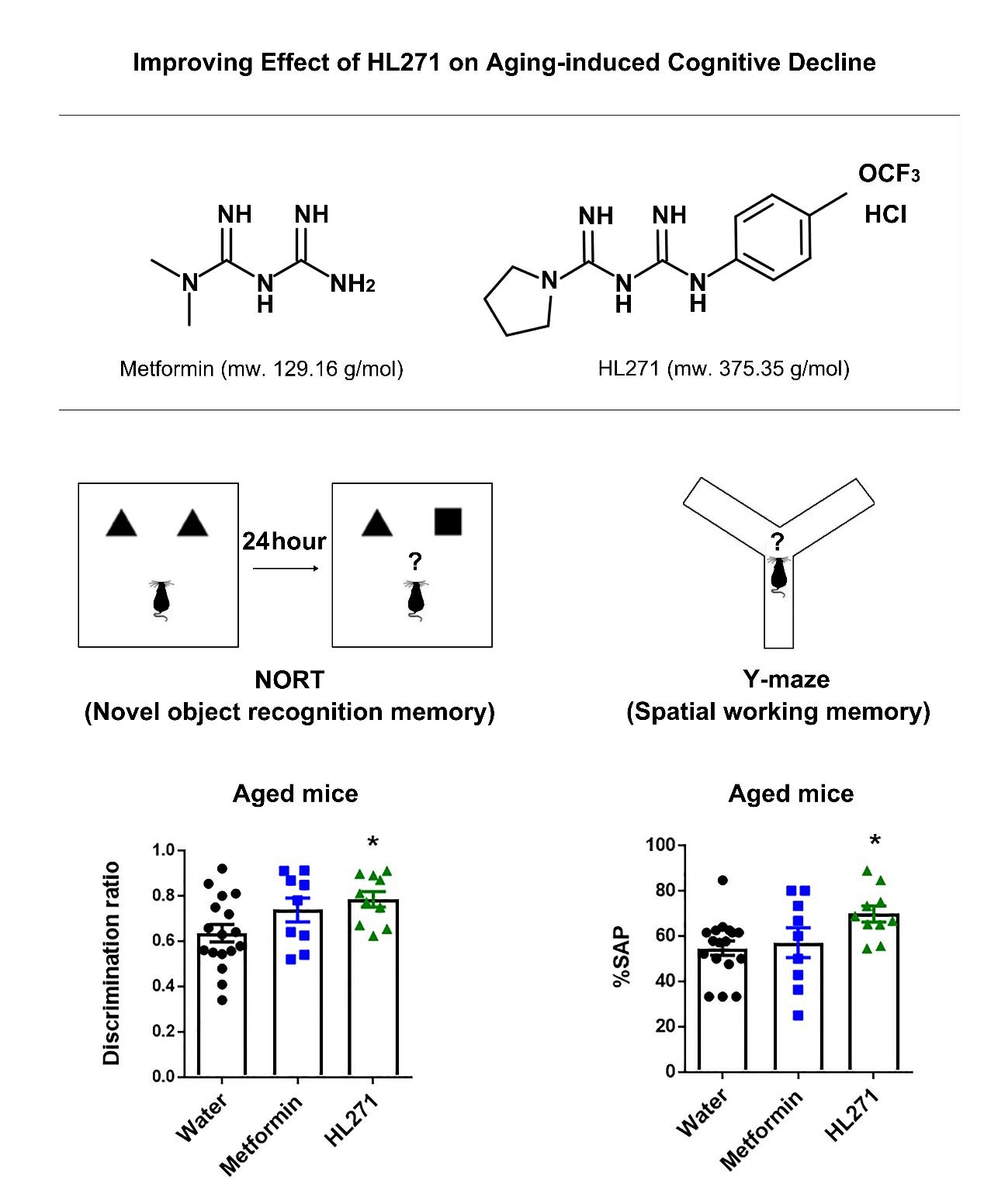
The Improving Effect of HL271, a Chemical Derivative of Metformin, a Popular Drug for Type II Diabetes Mellitus, on Aging-induced Cognitive Decline
Eunyoung Bang1,2†, Boyoung Lee1†, Joon-Oh Park3,4†, Yooncheol Jang1,3, Aekyong Kim5, Sungwuk Kim5,6 and Hee-Sup Shin1,2*
1Center for Cognition and Sociality, Institute for Basic Science, Daejeon 34141, 2Basic Science, IBS School, University of Science and Technology, Daejeon 34113, 3Center for Neuroscience, Korea Institute of Science and Technology, Seoul 02792, 4Division of Insect Pests, National Institute of Forest Science, Seoul 02455, Korea, 5ImmunoMet, Texas Medical Center,Houston, TX 77021, USA, 6Hanall Biopharma Inc., Seoul 06170, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-42-861-7016, FAX: 82-42-861-7060
e-mail: shin@ibs.re.kr
†These authors contributed equally
Abstract
In recent years, as the aging population grows, aging-induced cognitive impairments including dementia and Alzheimer's disease (AD) have become the biggest challenges for global public health and social care. Therefore, the development of potential therapeutic drugs for aging-associated cognitive impairment is essential. Metabolic dysregulation has been considered to be a key factor that affects aging and dementia. Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a primary sensor of cellular energy states and regulates cellular energy metabolism. Metformin (1,1-dimethylbiguanide hydrochloride) is a well-known AMPK activator and has been widely prescribed for type 2 diabetes mellitus (T2DM). Since the incidence of T2DM and dementia increases with aging, metformin has been considered to be one of the most promising drugs to target dementia and its related disorders. To that end, here, we tested the efficacy of metformin and HL271, a novel metformin derivative, in aging-induced cognitive decline. Water (control), metformin (100 mg/kg) or HL271 (50 mg/kg) were orally administered to aged mice for two months; then, the mice were subjected to behavioral tests to measure their cognitive function, particularly their contextual, spatial and working memory. AMPK phosphorylation was also measured in the drug-treated mouse brains. Our results show that oral treatment with HL271 (50 mg/kg) but not metformin (100 mg/kg) improved cognitive decline in aged mice. AMPK activation was correlated with behavior recovery after aging-induced cognitive decline. Taken together, these results suggest that the newly synthesized AMPK activator, HL271, could be a potential therapeutic agent to treat age-related cognitive decline.
Graphical Abstract
Keywords: Aging, Cognitive decline, AMPK, Metformin, HL271
INTRODUCTION
World Population Prospects: the 2015 Revision (United Nations, 2015) reported that the population of older persons, those aged 60 years or over, has substantially increased in most countries, and this growth is expected to accelerate in the coming decades [1]. Another study also reported that by 2050, the world's population aged 60 years and older is projected to reach 2 billion, increasing from 900 million in 2015 [2]. Moreover, the population of those aged 80 years or over is growing even faster, and within this age group, approximately 50% to over 60% are estimated to have dementia [3]. Therefore, in recent years, aging-induced cognitive impairments including dementia and Alzheimer's disease (AD) have become the biggest challenges for global public health and social care. Therefore, the development of potential therapeutic drugs for aging-associated cognitive impairment is essential.
Metabolic dysregulation is a key factor that affects both aging and dementia [4,5]. Indeed, the incidence of both T2DM, a metabolic disorder, and dementia increases with aging, and the prevalence of mild cognitive impairment (MCI) and dementia, including AD, is higher in individuals with diabetes [6,7]. Increased blood glucose levels in T2DM patients has been widely accepted to lead to medical complications, including negative influences on cognition [8,9,10]. Therefore, the use of antidiabetic medications for the management of various cognitive dysfunctions has received more attention. Some oral antidiabetic medications have been evaluated for their efficacy in improving cognitive function in MCI and AD patients [11]. Intranasal insulin treatment has also been shown to improve the memory and cognitive abilities of patients with MCI and AD [12,13]. Whether diabetes management can reduce the incidence of MCI and AD in the elderly remains uncertain; however, emerging lines of evidence suggest that diabetes therapies may have the potential to improve cognitive function [14,15,16].
For a recently initiated clinical trial called “Targeting Aging with Metformin” (TAME) supported by an R24 grant from the National Institute on Aging (NIA; J. Kirkland, N.B., S. Austad), Barzilai and colleagues chose metformin to target aging and its diseases including dementia and AD in humans for several reasons [17]. Metformin modulates metabolic and cellular processes including mitochondrial function [18], which is closely associated with the development of age-related conditions. Human clinical trials and observational studies have shown that metformin treatment is also beneficial for other age-related diseases such as cardiovascular disease (CVD) [19] and cancer [20]. More importantly, a growing body of evidence suggests that metformin is associated with a reduction in cognitive impairments in both T2DM patients [21] and non-diabetic subjects [22]. Furthermore, several studies have reported an association between metformin and decreased mortality [23]. In addition to those positive aspects of metformin in aging, metformin has been used with an outstanding safety record for over 60 years [17]. Thus, metformin is now seen as one of the most promising drugs to target aging and its associated diseases including dementia and AD. However, no definitive trials have been conducted to evaluate the efficacy of metformin in cognitive dysfunction in the elderly [17]. Therefore, separate studies should be performed to verify the effectiveness of metformin in attenuating cognitive impairments in the elderly and to understand related cellular mechanisms.
Metformin belongs to a class of drugs called biguanides, an organic compound with the formula HN(C(NH)NH2)2 and is currently the most widely prescribed drug for the treatment of type 2 diabetes [24,25]. The commonly accepted mechanism underlying the anti-hyperglycemic effects of metformin is the suppression of hepatic glucose production via the activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK), the primary energy sensor and regulator of energy homeostasis [26,27]. AMPK is a highly conserved serine/threonine protein kinase consisting of a catalytic α subunit and two regulatory β and γ subunits and is activated in response to an increase in the AMP: adenosine triphosphate (ATP) ratio in metabolic stress conditions, such as hypoxia, ischemia or glucose deprivation [28]. Several studies have provided evidence that metformin can slow or improve cognitive function decline [22]; however, whether metabolic control by metformin contributes to its beneficial effects on cognitive function remains unclear. Some studies have failed to confirm that cognition is improved in T2DM patients even after good glycemic control [29]. In a study by Mussell et al. [30], despite near normoglycemia levels for over 3 months in diabetic subjects, no specific effects on cognitive performance were observed when compared to controls. These findings suggest that glycemic control might not be essential for the beneficial effect of metformin on cognitive performance in patients.
Recently, Row et al. [31], investigated the effect of a novel metformin derivative, HL271, on molecular oscillations of clock genes and metabolic regulation in a diet-induced obesity mouse model and compared these effects with those of metformin. A time-course experiment showed that HL271, which phosphorylates AMPK, significantly increased AMPK phosphorylation levels faster and at much lower concentrations than metformin. Although HL271 acts by activating AMPK to regulate the circadian period and clock gene expression, HL271 did not affect the expression of key factors involved in glucose homeostasis such as glucose-6-phosphatase (G6pase) or phosphoenolpyruvate carboxykinase 1 (Pck1). Moreover, HL271 did not affect metabolic regulation assessed by body weight, blood glucose, insulin levels and lipid metabolite content in mice with diet-induced obesity, whereas metformin treatment significantly reduced all of them. In sum, HL271 is a potent AMPK activator, but its physiological effects are different from those of metformin, specifically regarding metabolic control [31].
Here, we utilized both metformin and HL271 for the behavioral assessment of cognitive function in mouse models of aging. Body weight changes before and two months after drug treatment as well as general locomotion and anxiety were measured. For cognitive function, spatial working memory, novel object recognition memory and contextual memory were evaluated. Tissues were collected at the end of these studies to measure AMPK enzyme activity levels.
MATERIALS AND METHODS
Animal care and experimental procedures followed the guidelines of the Institutional Animal Care and Use Committee of the Institute of Basic Science (IBS). Experiments were performed with male and female C57BL/6J mice (12~16 weeks of age for the young group and 20~22 months of age for the old groups). Group-housed mice were separated into single cages for acclimatization one month before beginning drug treatment. Mice were housed under controlled temperature and light conditions (23℃, 12-h light:12-h dark cycle). Experiments were performed during the light phase. The old mice were divided into three groups and orally treated with normal drinking water (control), metformin solution (100 mg/kg), or HL271 solution (50 mg/kg) for 2 months.
OFT was performed as previously reported [32]. Briefly, mice were placed in the center of an open field chamber (40×40×40 cm3) illuminated with 10 lux light and remained in the box for 30 min. After the test, each mouse was returned to its home cage, and the box was wiped clean using 70% ethanol and distilled water. Mouse activity was recorded and analyzed by using Ethovision 9.0 (Noldus Information Technology). Total distance moved for the 30 min and the duration spent in the center area for the first 5 min were analyzed to determine general locomotion and anxiety.
NORT was performed using previously described methods [33] with minor modifications. NORT was performed in the same box as the open field test (40×40×40 cm3); the box was illuminated with 10 lux light. On the first day of the experiment, the sample phase, two identical objects were presented to each mouse. The mouse was placed on one side of the open field box and allowed to freely explore for 20 min. After 24 hours, the test phase was conducted; in this phase, one of the old objects was replaced by a novel object and presented to each mouse for 10 min. To remove olfactory stimuli, chamber and objects were cleaned with 70 % ethanol and distilled water after testing each animal. The results are expressed as a discrimination ratio of the time spent with the novel object to the total exploration time.
Spatial working memory was assessed by using the Y-maze spontaneous alternation test as previously described with minor modifications [34]. The Y-maze consists of three arms of equal size. The arms were 32.5 cm long, 3 cm wide and 15 cm high. The Y-maze test was performed under 10 lux lighting conditions. Each mouse was placed at the end of one arm and was free to explore the arena for 8 min. A mouse was considered to make a triad when it entered all 3 arms consecutively. The maze was cleaned thoroughly with 70 % ethanol solution and distilled water between tests. As a measure of working memory, the percentage of spontaneous alternation performance (%SAP) was calculated as the number of triads divided by the maximum possible alternations (total number of arm entries -2) X 100.
CFC was performed as previously described [32] with minor modifications. Training consisted of placing the mice in the chamber for a period of 180 s, after which a 1 s foot shock (0.5 mA) without an auditory tone was delivered through the rod floor. Mice were returned to their home cage 60 s after the shock ended. For hippocampal-dependent memory tests, 24 hours after fear conditioning, mice were placed in the same conditioning context for five min. Freezing was assessed automatically using EthoVision 9.0 software (Noldus Information Technology). Freezing was defined as lack of any movement other than respiration and heartbeat for a 1 s interval and is presented as a percentage of the total test time.
For diaminobenzidine-based immunohistochemical labeling, free-floating sections were initially treated with 0.3 % H2O2 diluted in phosphate-buffered saline (PBS) to inhibit endogenous peroxidase activity and then blocked (30 min) with 5 % normal goat serum diluted in PBS with 0.1% Triton X-100 (PBST). Sections were then incubated overnight with an antibody against AMP-activated kinase (p-AMPK alpha (T172), rabbit monoclonal, 1;10,000, Cell Signaling). After sections were incubated with a biotinylated secondary antibody, the tissues were processed using the ABC labeling technique (Vector Labs, Burlingame, CA, USA), and nickel-intensified diaminobenzidine was used to visualize the signal. Sections were then mounted, dehydrated in xylene, and sealed with Permount (Sigma). Images were acquired using a Nikon inverted microscope (Nikon, Eclipse Ti), and data analyses were performed with ImageJ. The dorsal hippocampus was selected to measure intensity. The intensity values of the corpus callosum were then measured and subtracted as the background intensity. Values from 2 hemispheres from the dorsal hippocampus were averaged, and 3 consecutive sections from each mouse were averaged. A total of 4 to 5 mice from each group were averaged for statistical analyses.
RESULTS
Before we tested the efficacy of the AMPK activators metformin and HL271 in inhibiting age-associated cognitive decline, we needed to confirm that C57BL/6J mice, a common laboratory mouse strain, showed significant cognitive decline with age. To that end, we divided C57BL/6J mice into two groups, young (3~4 months) and aged (20~22 months) mice, and performed various memory tasks, such as the novel object recognition test (NORT), Y-maze and contextual fear conditioning (CFC). Before the memory tasks, the mice were subjected to an open field test (OFT) to observe their general behaviors, such as spontaneous locomotion and anxiety. The total distance the mice freely explored in the open-field arena was measured as its spontaneous locomotion, and the time spent in the center of the arena for the first 5 min of the OFT was measured as its anxiety level. The results showed no significant differences between young and aged mice for both locomotion (student's t-test; t=0.3346, df=30, p=0.7402; Fig. 1A) and anxiety (student's t-test; t=1.12, df=30, p=0.2716; Fig. 1B), indicating that the motor activity and anxiety levels of the aged mice in our study were within a normal range.
To test novel object recognition memory, mice were first placed in an open arena containing two of the same objects (sample phase). Twenty-four hours later (test phase), one of the familiar objects was replaced with a novel object to evaluate novel object recognition memory, a natural exploratory behavior in rodents. The aged mice explored the novel object significantly less than the young mice, indicating that recognition memory in the aged mice was significantly decreased (student's t-test; t=2.693, df=30, *p=0.0115; Fig. 1C). In the Y-maze task, when the percentage of alternations per group was compared to the chance level of 50%, both groups performed better than the chance level, which was indicative of functional working memory. Although aged mice had functional working memory, their spatial working memory was significantly attenuated compared to that of the young group (student's t-test; t=2.345, df=30, *p=0.0259; Fig. 1D). Regarding contextual fear memory, the freezing level (%) during the contextual retrieval test 24 hours after the conditioning represents the successful retention of hippocampal-dependent contextual fear memory. There was no significant difference in the freezing levels between the young and aged mice in the 24-h memory test, suggesting that there were no significant age-related impairments in contextual fear memory in the aged group (student's t-test; t=1.008, df=30, p=0.3216; Fig. 1E). Altogether, these results indicate that the C57BL/6J strain used in our study clearly showed an age-associated cognitive decline in novel object recognition memory and spatial working memory, suggesting that our age grouping and behavior schemes were appropriate for studying the efficacy of AMPK activators.
In a separate experimental series, the groups of aged mice were treated with normal drinking water (control group), metformin (Fig. 2A, 100 mg/kg) or HL271 (Fig. 2A, 50 mg/kg) for up to 2 months. The average water intake was no different among all three groups (one-way ANOVA; F(2, 28)=1.418, p=0.2590; Fig. 2B). We adjusted the drug dosage according to the average water intake during the habituation period. The changes in body weights were not significantly different among the three groups (one-way ANOVA; F(2, 28)=2, p=0.1543; Fig. 2C). As shown in Fig. 3A and 3B, neither the locomotor activity (one-way ANOVA; F(2,33)=0.4071, p=0.6687) nor anxiety (one-way ANOVA; F(2, 33)=0.4386, p=0.6486) were significantly different among the three groups during the OFT.
During the novel object recognition task, the discrimination ratio in the HL271-treated mice group was higher than that in the mice treated with water, indicating that HL271 treatment improved recognition memory in the aged mice (one-way ANOVA; F (2, 33)=3.655, *p=0.0368; Fig. 3C). For the spontaneous alternation task in the Y-maze, HL271 treatment significantly enhanced the spatial working memory compared to treatment with water (one-way ANOVA; F (2, 33)=3.52; *p=0.0412, Fig. 3D). Interestingly, in the CFC task, the freezing level was significantly increased in the HL271-treated group compared to that in the water-treated group (one-way ANOVA; F (2, 33)=5.17; *p=0.0111, Fig. 3E). Since there was no significant difference between the young and aged groups during the contextual fear memory retrieval test (student's t-test; p=0.3216; Fig. 1E), the enhancement of contextual fear memory by HL271 indicates that this drug improved memory in the aged mouse group to some degree.
Following the behavioral assays, immunohistochemistry was performed to measure AMPK activation. The hippocampus has been suggested to be associated with synaptic plasticity and memory, including spatial working [35], novel object recognition [36] and contextual fear memories [37]. Immunohistochemical analyses revealed that AMPK activation in the hippocampus was significantly lower in the aged mice than in the young mice (student's t-test; t=3.254, df=6, *p=0.0174; Fig. 4A and C). When the AMPK activators metformin and HL271 were administered to the aged mice, both the metformin and HL271 treatment groups expressed greater levels of AMPK phosphorylation than the water group, but this change was only significant in the HL271 treatment group (one-way ANOVA; F(2,34)=3.69, *p=0.0354; Fig. 4B and C). These results suggest that AMPK activation might be involved in the mechanism by which treatment with 50 mg/kg HL271 improved the aging-induced cognitive decline.
DISCUSSION
In this study, we tested the efficacy of two different AMPK activators, metformin and HL271, on aging-induced cognitive decline. The current study used the Y-maze and NORT, which clearly depicted age-associated declines in spatial working memory and novelty recognition memory in aged mice. The drug treatment assays revealed that 50 mg/kg HL271 not 100 mg/kg metformin significantly inhibited the aging-induced cognitive decline. In addition, HL271 enhanced hippocampal-dependent contextual memory. Altogether, our data suggest that HL271 is a potential therapeutic agent to treat aging-associated cognitive impairment or related diseases, such as dementia and AD.
Aging mice have been extensively used for investigating age-dependent memory decline and its underlying mechanisms and for developing drugs that potentially target aging and aging-associated cognitive dysfunctions. Several studies have shown extensive impairments in aging mice, including impairments in spatial working memory [38,39,40], object recognition memory [41], contextual memory [42,43] and motor coordination [44,45,46]. We found that C57BL/6J mice clearly showed impairments in novelty recognition memory (Fig. 1C) and spatial working memory (Fig. 1D) according to behavior tests at 20~22 months of age. However, we did not see any differences in locomotion (Fig. 1A), anxiety (Fig. 1B) or fear-related contextual memory (Fig. 1E) between the young (3~4 months) and aged (20~22 months) mouse groups . Although many researchers have reported impairments in locomotion and fear-related behaviors, several studies did not find significant differences in these behaviors, as we observed in this study [47,48]. Thus, a possible explanation for these behavior discrepancies is that the environment, food, bedding or housing may affect the general behaviors of animals maintained in a facility. However, we found significant impairments in spatial working memory and novel object recognition memory in the aged group compared to those in the young group, suggesting that our age grouping and behavior schemes were appropriate for studying the efficacy of drugs in the treatment of aging-induced cognitive decline.
Because cognitive impairment and T2DM are common disorders in the elderly, metformin and other diabetes medications have been tested in both humans and rodents to determine the potential efficacy of these drugs in the treatment of aging-related cognitive function decline [49,50]. Particularly, metformin (100 mg/kg) has been used to improve longevity, muscle tone and cognitive function without any adverse effects on organs and the brain [51,52,53]. However, the efficacy of metformin in improving cognitive function is still controversial. Some studies have shown that administration of metformin rather increased the risk of cognitive impairments [54,55,56]. A recent study [56] reported a deleterious effect of metformin on spatial memory and visual acuity. In that study, mice received metformin at higher doses (~219~297 mg/kg/day) and for a longer duration (3 months) than the mice in our study (100 mg/kg/day and 2 months). In this study, we observed neither deleterious effects nor beneficial effects of metformin on cognitive function (Fig. 3C~E). Only HL271 (50 mg/kg) showed significant effects on the aging-induced cognitive decline. Since HL271 is known to activate AMPK, we investigated the expression of phosphorylated AMPK two months after oral treatment with each drug and determined that treatment with 50 mg/kg HL271 yielded a higher level of AMPK phosphorylation in the hippocampus than treatment with 100 mg/kg metformin (Fig. 4B). This observation indicates that the improvement in cognitive function induced by HL271 in the aged mouse group might be AMPK-dependent. However, HL271 may also act in an AMPK-independent manner. Although there are no reports that have investigated the AMPK-independent effects of HL271, metformin is known to regulate mitogen signaling (mTORC1 and ERK) and DNA synthesis in pancreatic cancer cells through dose-dependent AMPK-dependent and -independent pathways [57]. Metformin also regulates adipogenesis through AMPK-dependent and -independent mechanisms [58]. Therefore, more research is needed to identify whether the improving effect of HL271 on aging-induced cognitive decline is through AMPK-dependent action. An extended study using AMPK inhibition, such as through hippocampal tissue-specific AMPK knockout or expression of a dominantnegative (DN) AMPK, along with HL271 treatment will be crucial to show a direct causal relationship between AMPK activity and the beneficial effect of HL271 on aging-induced cognitive decline.
What are possible reasons for the differences in the effects of HL271 and metformin on cognitive function in the aged mouse group? Many researchers consider aging-induced metabolic dysregulation to be a key factor that initiates cognitive decline. Therefore, extensive studies have focused on drugs that regulate or are linked to metabolism or associated pathways for treating dementia. Therefore, metformin has been considered as a potential drug due to its pleiotropic effects on metabolism, including sensitization to insulin, increase in glucose uptake, and decrease in hepatic glucose synthesis [59]. However, in this study, we found that HL271 was more effective than metformin at attenuating aging-induced cognitive decline. Currently, there is limited knowledge regarding the underlying mechanisms of the effects of HL271 on cognitive function. According to the recent results showing that, unlike metformin, HL271 is not involved in metabolic control [31], we considered that metabolic regulation might not be the important factor necessary to improve cognitive function in the aged mouse group. As a more potent AMPK activator, one possible mechanism of HL271 on cognitive function may be through activation of AMPK pathways that are not restricted to the maintenance of energy metabolism. In fact, several studies have shown that AMPK activation can coordinate autophagocytosis of damaged structures [60], increase tissue resistance against stressors such as oxidative stress [61] and endoplasmic stress [62] and suppress inflammatory disorders [63]. These processes controlled by AMPK activation are important for the regulation of the aging process [64] and cognitive function [65,66]. Interestingly, HL271 induced a greater increase in AMPK phosphorylation than metformin in the aged mouse group (Fig. 4B), and deficiency in the sensitivity of AMPK activation in aged tissues has been confirmed with different types of AMPK activators [67,68]. Therefore, unknown upstream signaling pathways might be uniquely activated by HL271 and not by metformin to disinhibit systems suppressing AMPK activation in aged tissues, i.e., protein phosphatase 2A (PP2A), PP2Ca and protein phosphatase, or Mg2+/Mn2+-dependent 1E (Ppm1E) [64]. In addition, distinct AMPK-independent signaling pathways are also possibly activated by HL271, which is as yet unclear. Therefore, future studies are required to identify the underlying cellular and molecular mechanisms of the HL271-mediated improvement in cognitive function in the aged mouse. Although we did not see a significant improvement in cognitive function with metformin treatment, we observed a tendency for metformin to inhibit the impairments in novel object recognition memory (Fig. 3C) and to enhance contextual fear memory (Fig. 3E). Therefore, larger sample sizes are necessary to draw more concrete conclusions.
Altogether, our study demonstrates the outstanding effects of the newly synthesized AMPK activator HL271 on aging-induced cognitive decline and raises the possibility of using HL271 as a future treatment for cognitive impairment and related diseases, such as AD and dementia.
Figures


References
- United Nations, Department of Economic and Social Affairs, Population Division. World population ageing 2015 (ST/ESA/SER.A/390). New York, NY: United Nations, 2015.
- World Health Organization. World report on ageing and health: fact sheet N°404. Geneva: World Health Organization, 2015.
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. N Engl J Med 2009;360:2302-2309.
- Ruiz HH, Chi T, Shin AC, Lindtner C, Hsieh W, Ehrlich M, Gandy S, Buettner C. Increased susceptibility to metabolic dysregulation in a mouse model of Alzheimer's disease is associated with impaired hypothalamic insulin signaling and elevated BCAA levels. Alzheimers Dement 2016;12:851-861.
- Tan ZS, Beiser AS, Fox CS, Au R, Himali JJ, Debette S, Decarli C, Vasan RS, Wolf PA, Seshadri S. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care 2011;34:1766-1770.
- Dash SK. Cognitive impairment and diabetes. Recent Pat Endocr Metab Immune Drug Discov 2013;7:155-165.
- Fagot-Campagna A, Bourdel-Marchasson I, Simon D. Burden of diabetes in an aging population: prevalence, incidence, mortality, characteristics and quality of care. Diabetes Metab 2005;31:S35-S52.
- Patiño-Fernández AM, Delamater AM, Applegate EB, Brady E, Eidson M, Nemery R, Gonzalez-Mendoza L, Richton S. Neurocognitive functioning in preschool-age children with type 1 diabetes mellitus. Pediatr Diabetes 2010;11:424-430.
- Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes 1996;45:1259-1266.
- Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, Coker LH, Murray A, Sullivan MD, Marcovina SM, Launer LJ, Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Investigators. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 2009;32:221-226.
- Beeri MS, Schmeidler J, Silverman JM, Gandy S, Wysocki M, Hannigan CM, Purohit DP, Lesser G, Grossman HT, Haroutunian V. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology 2008;71:750-757.
- Freiherr J, Hallschmid M, Frey WH, Brünner YF, Chapman CD, Hölscher C, Craft S, De Felice FG, Benedict C. Intranasal insulin as a treatment for Alzheimer's disease: a review of basic research and clinical evidence. CNS Drugs 2013;27:505-514.
- Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 2007;32:239-243.
- Alipour M, Adineh F, Mosatafavi H, Aminabadi A, Monirinasab H, Jafari MR. Effect of chronic intraperitoneal aminoguanidine on memory and expression of Bcl-2 family genes in diabetic rats. Can J Physiol Pharmacol 2016;94:669-675.
- Sircar M, Bhatia A, Munshi M. Review of hypoglycemia in the older adult: clinical implications and management. Can J Diabetes 2016;40:66-72.
- Alagiakrishnan K, Sankaralingam S, Ghosh M, Mereu L, Senior P. Antidiabetic drugs and their potential role in treating mild cognitive impairment and Alzheimer's disease. Discov Med 2013;16:277-286.
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab 2016;23:1060-1065.
- Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr 2006;38:33-42.
- Kooy A, de Jager J, Lehert P, Bets D, Wulffelé MG, Donker AJ, Stehouwer CD. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616-625.
- Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7:867-885.
- Cheng YY, Leu HB, Chen TJ, Chen CL, Kuo CH, Lee SD, Kao CL. Metformin-inclusive therapy reduces the risk of stroke in patients with diabetes: a 4-year follow-up study. J Stroke Cerebrovasc Dis 2014;23:e99-e105.
- Luchsinger JA, Perez T, Chang H, Mehta P, Steffener J, Pradabhan G, Ichise M, Manly J, Devanand DP, Bagiella E. Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis 2016;51:501-514.
- Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 2014;16:1165-1173.
- Rojas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 2013;5:6.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668-2675.
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab 2014;20:953-966.
- Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res 2012;53:2490-2514.
- Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 2003;144:5179-5183.
- Hewer W, Mussell M, Rist F, Kulzer B, Bergis K. Short-term effects of improved glycemic control on cognitive function in patients with type 2 diabetes. Gerontology 2003;49:86-92.
- Mussell M, Hewer W, Kulzer B, Bergis K, Rist F. Effects of improved glycaemic control maintained for 3 months on cognitive function in patients with type 2 diabetes. Diabet Med 2004;21:1253-1256.
- Row H, Jeong J, Cho S, Kim S, Kim K. HL271, a novel chemical compound derived from metformin, differs from metformin in its effects on the circadian clock and metabolism. Biochem Biophys Res Commun 2016;469:783-789.
- Keum S, Park J, Kim A, Park J, Kim KK, Jeong J, Shin HS. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav 2016;15:231-242.
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T. Object recognition test in mice. Nat Protoc 2013;8:2531-2537.
- Wolf A, Bauer B, Abner EL, Ashkenazy-Frolinger T, Hartz AM. A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS One 2016;11:e0147733.
- Sanderson DJ, Good MA, Skelton K, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Enhanced long-term and impaired short-term spatial memory in GluA1 AMPA receptor subunit knockout mice: evidence for a dual-process memory model. Learn Mem 2009;16:379-386.
- Diaz A, Treviño S, Vázquez-Roque R, Venegas B, Espinosa B, Flores G, Fernández-G JM, Montaño LF, Guevara J. The aminoestrogen prolame increases recognition memory and hippocampal neuronal spine density in aged mice. Synapse 2017;71:e21987.
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 1992;106:274-285.
- Magnusson KR, Scruggs B, Aniya J, Wright KC, Ontl T, Xing Y, Bai L. Age-related deficits in mice performing working memory tasks in a water maze. Behav Neurosci 2003;117:485-495.
- Lebrun C, Durkin TP, Marighetto A, Jaffard R. A comparison of the working memory performances of young and aged mice combined with parallel measures of testing and drug-induced activations of septo-hippocampal and nbmcortical cholinergic neurones. Neurobiol Aging 1990;11:515-521.
- Bernstein D, Olton DS, Ingram DK, Waller SB, Reynolds MA, London ED. Radial maze performance in young and aged mice: neurochemical correlates. Pharmacol Biochem Behav 1985;22:301-307.
- Fahlström A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging 2011;32:1868-1880.
- Sanders MJ. Context processing in aging: older mice are impaired in renewal of extinguished fear. Exp Aging Res 2011;37:572-594.
- Frye CA, Walf AA. Progesterone enhances performance of aged mice in cortical or hippocampal tasks. Neurosci Lett 2008;437:116-120.
- Ingram DK, London ED, Reynolds MA, Waller SB, Goodrick CL. Differential effects of age on motor performance in two mouse strains. Neurobiol Aging 1981;2:221-227.
- Ingram DK, London ED, Waller SB, Reynolds MA. Age-dependent correlation of motor performance with neurotransmitter synthetic enzyme activities in mice. Behav Neural Biol 1983;39:284-298.
- Miquel J, Blasco M. A simple technique for evaluation of vitality loss in aging mice, by testing their muscular coordination and vigor. Exp Gerontol 1978;13:389-396.
- Boger HA, Mannangatti P, Samuvel DJ, Saylor AJ, Bender TS, McGinty JF, Fortress AM, Zaman V, Huang P, Middaugh LD, Randall PK, Jayanthi LD, Rohrer B, Helke KL, Granholm AC, Ramamoorthy S. Effects of brain-derived neurotrophic factor on dopaminergic function and motor behavior during aging. Genes Brain Behav 2011;10:186-198.
- Wiklund A, Granon S, Cloëz-Tayarani I, Faure P, le Sourd AM, Sundman E, Changeux JP, Eriksson LI. Sevoflurane anesthesia alters exploratory and anxiety-like behavior in mice lacking the beta2 nicotinic acetylcholine receptor subunit. Anesthesiology 2008;109:790-798.
- Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer's disease: shared pathology and treatment?. Br J Clin Pharmacol 2011;71:365-376.
- Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn Mem 2014;21:119-126.
- Oliveira WH, Nunes AK, França ME, Santos LA, Lós DB, Rocha SW, Barbosa KP, Rodrigues GB, Peixoto CA. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res 2016;1644:149-160.
- Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 2012;11:23-35.
- Patrone C, Eriksson O, Lindholm D. Diabetes drugs and neurological disorders: new views and therapeutic possibilities. Lancet Diabetes Endocrinol 2014;2:256-262.
- Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer's disease. J Neurochem 2011;118:460-474.
- Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Woodward M, Boundy K, Ellis KA, Bush AI, Faux NG, Martins R, Szoeke C, Rowe C, Watters DA, AIBL Investigators. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 2013;36:2981-2987.
- Thangthaeng N, Rutledge M, Wong JM, Vann PH, Forster MJ, Sumien N. Metformin impairs spatial memory and visual acuity in old male mice. Aging Dis 2017;8:17-30.
- Ming M, Sinnett-Smith J, Wang J, Soares HP, Young SH, Eibl G, Rozengurt E. Dose-dependent AMPK-dependent and independent mechanisms of berberine and metformin inhibition of mTORC1, ERK, DNA synthesis and proliferation in pancreatic cancer cells. PLoS One 2014;9:e114573.
- Chen SC, Brooks R, Houskeeper J, Bremner SK, Dunlop J, Viollet B, Logan PJ, Salt IP, Ahmed SF, Yarwood SJ. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol Cell Endocrinol 2017;440:57-68.
- Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A 2009;106:3907-3912.
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011;13:1016-1023.
- Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, Coselli JS, Chen L, Wang XL, Zhang Y, Shen YH. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes 2009;58:2246-2257.
- Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation 2010;121:792-803.
- Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 2011;89:667-676.
- Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 2012;11:230-241.
- Coronas-Samano G, Baker KL, Tan WJ, Ivanova AV, Verhagen JV. Fus1 KO mouse as a model of oxidative stress-mediated sporadic Alzheimer's disease: circadian disruption and long-term spatial and olfactory memory impairments. Front Aging Neurosci 2016;8:268.
- Poulose SM, Bielinski DF, Carey A, Schauss AG, Shukitt-Hale B. Modulation of oxidative stress, inflammation, autophagy and expression of Nrf2 in hippocampus and frontal cortex of rats fed with açaí-enriched diets. Nutr Neurosci 2017;20:305-315.
- Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 2007;5:151-156.
- Ljubicic V, Hood DA. Diminished contraction-induced intracellular signaling towards mitochondrial biogenesis in aged skeletal muscle. Aging Cell 2009;8:394-404.



