Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2019; 28(2): 289-299
Published online March 20, 2019
https://doi.org/10.5607/en.2019.28.2.289
© The Korean Society for Brain and Neural Sciences
Delayed Treatment of Capsaicin Produces Partial Motor Recovery by Enhancing Dopamine Function in MPP+-lesioned Rats via Ciliary Neurotrophic Factor
Kyoung In Kim1,†, Jeong Yeob Baek1,†, Jae Yeong Jeong1,†, Jin Han Nam1, Eun Su Park1, Eugene Bok3, Won-Ho Shin3*, Young Cheul Chung2*, and Byung Kwan Jin1,2*
1Department of Neuroscience, Graduate School, Kyung Hee University, Seoul 02447, Korea.
2Department of Biochemistry & Molecular Biology, School of Medicine, Kyung Hee University, Seoul 02447, Korea.
3Department of Predictive Toxicology, Korea Institute of Toxicology, Daejeon 34114, Korea.
Correspondence to: *To whom correspondence should be addressed.
Byung Kwan Jin, TEL: 82-2-961-9288, FAX: 82-2-969-4570, e-mail: bkjin@khu.ac.kr
Young Cheul Chung, TEL: 82-2-961-9288, FAX: 82-2-969-4570, e-mail: ychung01@khu.ac.kr
Won-Ho Shin, TEL: 82-42-610-8088, FAX: 82-42-610-8157, e-mail: whshin@kitox.re.kr
†These authors contributed equally to this work.
Abstract
Transient receptor potential vanilloid subtype 1 (TRPV1) on astrocytes prevents ongoing degeneration of nigrostriatal dopamine (DA) neurons in MPP+-lesioned rats via ciliary neurotrophic factor (CNTF). The present study determined whether such a beneficial effect of astrocytic TRPV1 could be achieved after completion of injury of DA neurons, rather than ongoing injury, which seems more relevant to therapeutics. To test this, the MPP+-lesioned rat model utilized here exhibited approximately 70~80% degeneration of nigrostriatal DA neurons that was completed at 2 weeks post medial forebrain bundle injection of MPP+. TRPV1 agonist, capsaicin (CAP), was intraperitoneally administered. CNTF receptor alpha neutralizing antibody (CNTFRαNAb) was nigral injected to evaluate the role of CNTF endogenously produced by astrocyte through TRPV1 activation on DA neurons. Delayed treatment of CAP produced a significant reduction in amphetamine-induced rotational asymmetry. Accompanying this behavioral recovery, CAP treatment increased CNTF levels and tyrosine hydroxylase (TH) activity in the substantia nigra pars compacta (SNpc), and levels of DA and its metabolites in the striatum compared to controls. Interestingly, behavioral recovery and increases in biochemical indices were not reflected in trophic changes of the DA system. Instead, behavioral recovery was temporal and dependent on the continuous presence of CAP treatment. The results suggest that delayed treatment of CAP increases nigral TH enzyme activity and striatal levels of DA and its metabolites by CNTF endogenously derived from CAP-activated astrocytes through TRPV1, leading to functional recovery. Consequently, these findings may be useful in the treatment of DA imbalances associated with Parkinson's disease.
Graphical Abstract
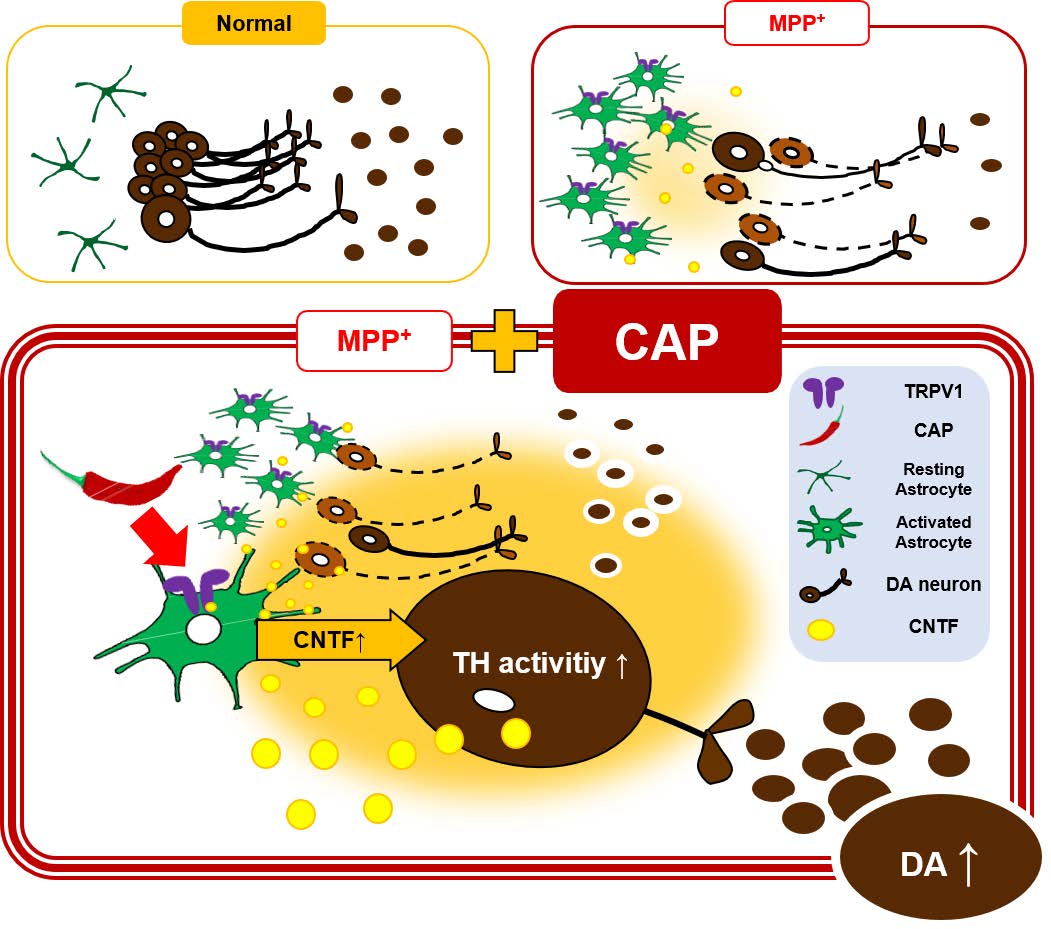
Keywords: Parkinson's disease, Astrocyte, Dopaminergic neuron, TRPV1, Ciliary neurotrophic factor
INTRODUCTION
Parkinson's disease (PD) is characterized by typical motor symptoms, such as tremor, muscle rigidity and bradykinesia, due to progressive loss of dopamine (DA) neurons in the substantia nigra pars compacta (SNpc) and DA depletion in the striatum (STR). To mimic PD symptoms by producing degeneration of midbrain DA neurons, animal models were established by administration of various neurotoxins, such as 6-hydroxydopamine (6-OHDA) [2], 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [3] or 1-methyl-4-phenylpyridinium (MPP+) [1], as well as genetic molecules, such as α-synuclein [1].
Transient receptor potential vanilloid subtype 1 (TRPV1) activated by capsaicin (CAP) is widely expressed in the brain, especially in DA neurons, as well as glial cells (microglia and astrocytes) in the SN
Neurotrophic factors (NTFs), among therapeutic compounds for midbrain DA neurons, are mainly delivered during ongoing degeneration of DA neurons (approximately ~50% loss of DA neurons) in a variety of PD animal models. Ciliary neurotrophic factor (CNTF) supports the maintenance of DA neurons in the substantia nigra [7,8] and motor neurons in spinal cord [9,10]. Given the importance of CNTF function in the central nervous system, CNTF is highlighted as a neurorestorative target for neurodegenerative diseases such as retinal disease [11] and Huntington's disease [12]. When exogenously delivered into the midbrain DA system, CNTF has potent neuroprotective effects for degenerating DA neurons in the SN
In the present study, using the complete medial forebrain bundle MPP+-lesion (completion of approximately 70~80% loss of nigrostriatal DA neurons at 2 weeks post MPP+), we show that delayed treatment of CAP at 9 weeks post MPP+ improved amphetamine-induced rotational asymmetry at 10 weeks post MPP+. Accompanying this behavioral recovery, CAP increased levels of CNTF and of serine-31-phosphorylated (active) TH (pTHser31) in the SN, as well as DA, dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) in the STR at 10 weeks post MPP+. The observed beneficial effects were reversed by CNTF alpha neutralizing antibody (CNTFRαNAb), indicative of CNTF involvement. Moreover, behavioral recovery and increases in biochemical indices did not reflect increased neuronal survival of the DA system. Of importance, behavioral recovery was transient and dependent on the continuous presence of TRPV1 activation by CAP.
MATERIALS AND METHODS
All experiments were done in accordance with Institutional Animal Care and Use Committee of Kyung Hee University and to minimize the number of animal experiments and suffering, we carried out the experiment with strict observance of the protocols and guidelines established by Kyung Hee University (KHUASP (SE)-16-059, 8 Aug 2016). Female Sprague-Dawley rats (10 weeks of age, 240~270 g, purchased from Daehan Biolink, introduced from Taconic Co., Albany, NY, USA) were housed under a 12:12 h (hour) light: dark cycle at an ambient temperature of 22℃. Water and rat chow were available ad libitum.
Stereotaxic surgery under chloral hydrate was performed as described [1,5,18]. Using coordinates relative to the bregma, stereotaxic injections of MPP+ (right medial forebrain bundle; A/P −3.6, M/L −2.0 D/V −7.7; MPP+, 7.4 µg in 2 µl phosphate-buffered saline, sigma) [1,5], or CNTFRαNAb antibody (right SN; A/P −5.5, M/L −2.2, D/V −7.7; R&D, AF-303-NA; 0.01 µg/µl, 0.2 µl/min, total 2µl) and respective control were done according to the atlas of Paxinos and Watson [18].
Capsaicin (1 mg/kg intraperitoneally; a single injection/day for 7 days, Sigma) [5,19] was injected at 4 or 9 week and 1 day post MPP+.
D-Amphetamine (5 mg/kg, intraperitoneally) was used to monitor ipsilateral rotations in rats with unilaterally lesioned nigrostriatal dopamine neurons [20]. The ipsilateral rotations were counted for 1 h at 3, 5, 8 or 10 weeks post MPP+.
As previously described [5,21,22,23], the total number of TH+ and Nissl+ cells was counted in the various animal groups using the optical fractionator method performed on a bright-field microscope (Olympus Optical, BX51) using Stereo Investigator software (MBF Bioscience). This unbiased stereological method of cell counting is not affected by either the reference volume (SNpc) or the size of the counted elements (neurons).
Optical densities of the TH+ striatal fiber were measured using Science Lab 2001 image Gauge (Fujifilm) [1].
Imaging data were analyzed in Image J (National Institutes of Health) as described recently [24]. Image J with co-localization plugin was used to quantify immunofluorescence and with color deconvolution plugin was used to quantify chromogenic signal intensity on image.
The SN area was rapidly removed and western blot analysis was performed as previously described [22,25]. The following primary antibodies and dilutions were used: rabbit TRPV1 (1:1000, Alomone labs), mouse anti-CNTF (1:1000, Millipore), mouse anti-GFAP (1:500, Sigma), mouse anti-tyrosine hydroxylase (TH, 1:1000, Millipore), rabbit anti-pTHser31 (1:1000, Millipore), and mouse anti-beta-actin (1:5000, Abcam). The following secondary antibodies and dilutions were used; horseradish peroxidase-conjugated anti- rabbit or mouse IgG (1:5000, Bethyl).
Animals were transcardially perfused, fixed and brain tissues (40 µm thick) and processed for immunohistochemical staining. In brief, sections were rinsed in phosphate-buffered saline (PBS) then incubated with the following primary antibodies: rabbit anti-TRPV1 (1:1000, Alomone labs) and rabbit anti-CNTF (1:200, Santa-Cruz), mouse anti-GFAP (1:500, mouse, Sigma) for astrocytes and rabbit anti-TH (1:2000, rabbit, Pel-Freez) for dopamine neurons. 3,3'-diaminobenzidine (DAB; Sigma) was used to visualize TH+ cell and fiber. 1% Cresyl violet (Sigma) solution was used for Nissl staining. The next day, tissues were rinsed and incubated with FITC-conjugated-anti-mouse goat IgG (1:400, Millipore) and/or Cy3-conjugated-anti-rabbit IgG (1:400, Millipore) for 1 h. The stained tissues were viewed using a confocal microscopy (LSM 700, Carl Zeiss) or were analyzed under a bright-field microscope (Olympus).
Levels of DA, DOPAC and HVA in STR were measured using the reversed-phase high performance liquid chromatography (HPLC,1260 Infinity system, Agilent Technologies, Santa Clara, CA) with electrochemical detector as described recently [26]. Dissected striatal tissues were homogenized with 0.1 M perchloric acid and 50 µM ascorbic acid per 10 mg brain tissue. Homogenate incubated on ice for 1h after sonication and centrifuged at 14000 rpm for 15 min, 4℃. the supernatants filtrated by 0.2 µm hydrophilic filter and injected into an auto-sampler at 4℃ (Waters 717 plus auto-sampler) and eluted through Sunfire C18 column (5 µm, 4.6×100 mm) with mobile phase for catecholamine analysis. The peaks of dopamine content were analyzed by ESA Coulochem III electrochemical detector (Dionex Corporation, Chelmsford, MA) and integrated using a commercially available program.
All values are expressed as mean ± standard error of the mean. Statistical significance (p<0.05 for all analysis) was assessed by One way ANOVA Newman-Keuls analyses and Student unpaired
RESULTS
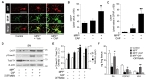
We first examined when MPP+ neurotoxicity could be complete. Immunohistochemical analysis demonstrated that MPP+ produces significant decreases in the number of TH+ and Nissl+ cells, assessed by stereology in the SNpc and density of TH+ fibers in the STR. When quantified and expressed as a percentage of cells on the MPP+-lesioned side compared to the PBS-treated control side, the number of TH+ and Nissl+ cells in the SNpc decreased by 68% and 63% at 2 weeks post MPP+, respectively (Fig. 1B, 1E, and 1J). Similar to those of 2 weeks, the number of TH+ and Nissl+ cells decreased by 78% and 65% at 10 weeks post MPP+, respectively (Fig. 1C, 1F, and 1J). In addition, the optical density of striatal fibers was significantly attenuated by 81% at 2 weeks and 82% at 10 weeks post MPP+ (Fig. 1H, 1I, and 1K). Taken together, the MPP+ model employed here showed a complete loss of nigrostriatal DA neurons at 2 weeks post MPP+ and no spontaneous recovery (restoration) of nigrostriatal DA neurons between 2 and 10 weeks post MPP+.
At 9 weeks post MPP+, rats which exhibited amphetamine-induced rotational asymmetry were randomly selected and treated with vehicle (Veh) as a control or CAP (i.p. 1 mg/kg; a continuous single injection per day for 7 days; Fig. 2A). At 10 weeks post MPP+, amphetamine-induced rotational asymmetry was significantly reduced in CAP-treated rats compared to Veh-treated rats (Fig. 2B). Next, we hypothesized that CAP effects on behavioral recovery are associated with neurorestoration on nigrostriatal DA neurons. Analysis by TH immunohistochemistry revealed that CAP treatment did not affect the number of TH+ cells, as assessed by stereology in the SNpc (Fig. 2C and 2D) and the density of TH+ fibers in the STR (Fig. 2C and 2E) in MPP+-lesioned rats compared to Veh-treated MPP+-lesioned control (Fig. 2C~E). Similar to TH immunohistochemical data, CAP treatment showed no substantial changes of Nissl+ cells compared to Veh treatment (Fig. 2D) in the SNpc of MPP+-lesioned rats compared to Veh-treated MPP+-lesioned control.
Our recent report demonstrates that CAP-induced behavioral recovery is attributable to endogenous production of CNTF, acting on CNTFRα expressed in DA neurons [1]. Accordingly, we tested the effects of CNTF and CNTFRα on behavioral recovery. CNTFRαNAb was unilaterally administered to inhibit CNTF actions in MPP+-lesioned SNpc at 9 weeks post MPP+ (Fig. 2A). CNTFRαNAb attenuated the effects of CAP on amphetamine-induced rotational asymmetry at 10 weeks post MPP+ (Fig. 2B), indicative of CNTF involvement.
Double immunofluorescence staining was performed on sections adjacent to those used for TH immunostaining in Fig. 1. Expression of TRPV1 in GFAP+ astrocytes was significantly increased in the SNpc at 2 weeks post MPP+, when MPP+ neurotoxicity was completed, and sustained up to 10 weeks post MPP+ compared with contralateral SNpc as a control (Fig. 3B and 3C). In a separate series of experiments on MPP+-lesioned rats, protein levels of TH, GFAP, and TRPV1 were measured at indicated time points (Fig. 3A). Western blot analysis demonstrated increases in GFAP expression with total TRPV1 levels unchanged in the SN, whereas TH levels were significantly decreased by 64% at 1 week, 81% at 2 weeks, and 80% at 10 weeks post MPP+, respectively, compared to the control (Fig. 3D and 3E). Taken together, these results indicate selective and prolonged expression of TRPV1 in GFAP+ astrocytes up to 10 weeks post MPP+.
As CNTF seems to be involved in CAP-induced behavioral recovery, and MPP+-lesion-induced increase in TRPV1 expression on astrocytes is sustained up to 10 weeks post MPP+. We wondered if CNTF could be endogenously produced by CAP-activated astrocytes through TRPV1 at 10 weeks post MPP+, leading to behavioral recovery. Immunohistochemical analysis revealed a significant increase in CNTF and CNTF expression in astrocytes in the SNpc of CAP-treated rats, compared to Veh-treated control at 10 weeks post MPP+ (Fig. 4A~C).
CNTF can stimulate TH activity via pTHser31 in the SN of MPP+-lesioned rats
As amphetamine-induced rotational asymmetry is highly correlated with striatal levels of DA and its metabolites (DOPAC and HVA) [20], we wondered if increases in nigral TH activity by CNTF could elevate striatal DA biochemistry. Analysis by HPLC revealed that, in CAP-treated MPP+-lesioned rats, striatal levels of DA, DOPAC, and HVA were increased by 114% (p<0.01), 121% (p<0.01), and 365% (p<0.001), respectively, compared to Veh-treated MPP+-lesioned rats (Fig. 4F). Moreover, treatment with CNTFRαNAb partially reverted striatal levels of DA, DOPAC, and HVA (Fig. 4F). It is therefore likely that, compared to Veh-treated control, the increase in levels of DA and its metabolites by CAP treatment may be responsible for amphetamine-induced behavioral recovery in MPP+-lesioned rats.
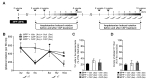
The long-term effects of delayed CAP treatment on behavioral recovery were also determined after completion of MPP+ neurotoxicity on DA neurons (between 2 weeks and 10 weeks, see Fig. 1). Rats that exhibited amphetamine-induced rotational asymmetry at 3 weeks post MPP+ were randomly selected and treated Veh as a control or CAP (i.p. 1 mg/kg; a continuous single injection per day for 7 days), starting at 4 weeks post MPP+ (Fig. 5A). At 5 weeks post MPP+, CAP attenuated amphetamine-induced rotational asymmetry compared to Veh-treated control (Fig. 5B). Intriguingly, after termination of the first CAP treatment, behavioral recovery almost reverted to control levels (rotational score similar to Veh-treated MPP+-lesioned rats) at 8 weeks post MPP+ (4 weeks after the first CAP treatment) (Fig. 5B), indicating that CAP effects were temporal and persisted for no more than 3 weeks.
Then, all rats (n=6) that received the first CAP treatment (at 4 weeks post MPP+, see Fig. 5A) were randomly selected and divided into two groups: one (n=3) that received Veh as a control, and the other (n=3) that received the second CAP treatment (i.p. 1 mg/kg; a continuous single injection per day for 7 days), starting at 9 weeks post MPP+, respectively (Fig. 5A). At 10 weeks post MPP+, rats receiving CAP exhibited a significant reduction in amphetamine-induced rotational asymmetry compared to those receiving Veh as a control (Fig. 5B). Similar to the results shown in Fig. 2, immunohistochemical analysis revealed that CAP had no trophic effects on DA neurons in the SNpc and their fibers in the STR (Fig. 5C and 5D). As an additional control, rats that received Veh at 4 and 9 weeks post MPP+, without CAP treatment, showed no reduction in amphetamine-induced rotational asymmetry at 5 and 10 weeks post MPP+, respectively (Fig. 5B). Taken together, the present data suggest that behavioral recovery by delayed CAP treatment might be transient without neurorestoration and require continuous TRPV1 activation by CAP.
DISCUSSION
The results of the present study describe the
We have recently shown that TRPV1 activation by capsaicin produces endogenous CNTF, which protects nigral DA neurons against degeneration of DA neurons and improves behavioral recovery in the partial MPP+-lesioned rat model of Parkinson's disease [1]. However, when diagnosed as PD, patients have already lost 50~80% of nigrostriatal DA neurons [21,22] and minimal or no TH innervation to putamen after 5 years of diagnosis [23], indicative of severe (complete) lesion. Despite this clinical feature of PD patients, spontaneous recovery of DA neurons occurred in the partial lesioned PD animal models [24,25]. Therefore, a completely lesioned PD animal model without spontaneous recovery seems to be necessary to mimic this clinical feature of PD patients [26,27]. Regarding this, the animal model employed here has a complete lesion of nigrostriatal DA neurons when starting CAP treatment at 9 weeks post MPP+, in that approximately 70~80% loss of TH+ cells in the SN and their fibers in the STR was already completed at 2 weeks post MPP+ and sustained up to 10 weeks post MPP+. Given our results that there was no spontaneous recovery in MPP+-lesioned SN, delayed treatment with CAP improved amphetamine-induced rotational behavior without neuroprotective or neurorestorative effects on DA neurons in completely lesioned rat model of PD. Taken together, the present findings may shed light on the new therapeutic strategy to treat PD.
Neurotrophic factors (NTFs) exogenously administered in completely lesioned PD animal models improved motor behavior with functional enhancement of the DA system in the midbrain. A reduction in apomorphine-induced rotational asymmetry and an increase in striatal DA levels were achieved by intranigral injection of adenoviral vector expressing GDNF (Ad-GDNF) at 10 weeks post 6-OHDA [27] or by intrastriatal injection of Ad-GDNF at 4 weeks post 6-OHDA [28]. Intrastriatal injection of adeno associated viral vector expressing CDNF (AAV8-CDNF) resulted in the reduction of apomorphine-induced rotational asymmetry and elevation of striatal DA levels at 5 weeks post 6-OHDA [29]. These studies also demonstrated that behavioral recovery is correlated with NTFs-induced trophic changes (restoration) of DA neurons. The number of TH+ cells in the SN and the density of TH+ fibers in the STR were significantly increased after intrastriatal injection of an adenoviral vector expressing GDNF (Ad-GDNF) at 4 weeks post 6-OHDA [28] or of adeno associated viral vector expressing CDNF (AAV8-CDNF) at 5 weeks post 6-OHDA [29]. In contrast, results obtained from complete 6-OHDA lesioned rats indicated that GDNF infusion decreased apomorphine-induced rotation without nigrostriatal DA neuron recovery (neurorestoration) [30]. This is in line with our present data, revealing that a reduction in amphetamine-induced rotational asymmetry by endogenous CNTF occurs without neurorestoration, as analyzed by TH immunohistochemistry (no changes in the number of TH+ cells in the SN and density of TH+ fibers in the STR).
Instead, endogenous CNTF increased nigral TH enzyme activity, as evidenced by upregulation of pTHser31 levels, leading to an increase in striatal DA levels and its metabolites, and subsequent behavioral recovery. This interpretation is supported by the findings that Ser-31 phosphorylation regulates TH subcellular localization by enabling its transport along microtubules toward the TH terminals in the STR [31]. It is therefore likely that increased levels of striatal pTHser31 by anterograde axonal transport may increase and maintain striatal levels of DA and its metabolites, which accounts for amphetamine-induced behavioral recovery [31,32,33]. We have shown that astrocytic TRPV1-derived CNTF rescues dopamine neuron against MPP+ neurotoxicity and inhibits microglial derived reactive oxygen species generation and oxidative damages through CNTFRα expressed in dopamine neurons [1] and microglia
It seems noteworthy that the reduction in amphetamine-induced rotational asymmetry by delayed CAP treatment was not permanent. Rather, it was dependent on the continuous presence of CAP treatment. As shown in Fig. 5, rats that received the first CAP treatment at 4 weeks post MPP+ exhibited amphetamine-induced behavioral recovery at 5 weeks post MPP+ (1 week after the first CAP treatment), compared to Veh-treated MPP+-lesioned control rats. In the absence of CAP, at 8 weeks post MPP+ (4 weeks after the first CAP treatment), however, all rats that exhibited CAP-induced behavioral recovery at 5 weeks post MPP+ (1 week after first CAP treatment) failed to show a reduction in amphetamine-induced rotational asymmetry. Rather, CAP effects were temporal, resulting in a reversion of amphetamine-induced behavioral recovery to control levels (rotation number of Veh-treated MPP+-lesioned rats) at 8 weeks post MPP+ (4 weeks after the first CAP treatment) (Fig. 5B). Among them, rats that received the second CAP treatment at 9 weeks post MPP+ improved amphetamine-induced rotational asymmetry at 10 weeks post MPP+ compared to Veh-treated MPP+-lesioned rats, which showed little changes of amphetamine-induced rotational asymmetry. Although the underlying mechanisms mediating these effects remain elusive, it is possible that prolonged expression of TRPV1 on astrocytes up to 10 weeks post MPP+ may be involved in transient behavioral recovery without the apparent presence of neurorestoration. This hypothesis is supported by findings consistent with our recent report [1], in which CAP treatment can activate TRPV1 on astrocytes and endogenously produce CNTF, which increases nigrostriatal DA function (TH enzyme activity in the SN and levels of DA and its metabolites in the STR) and improves amphetamine-induced rotational asymmetry. Taken together, the present data suggest that behavioral recovery by delayed CAP treatment may be temporal, which results from transient functional recovery of nigrostriatal DA neurons only in the presence of CAP treatment.
The present study reveals a new mechanism for behavioral recovery by delayed CAP treatment in a complete MPP+-lesioned rat model of PD. Prolonged expression of TRPV1 on astrocytes may be beneficial to regulate the endogenous production of CNTF by delayed CAP treatment, resulting in increased TH activity and levels of DA and its metabolites, and subsequent behavioral recovery in complete MPP+-lesioned rats. As expression of TRPV1 on astrocytes is significantly increased in the SN of human PD patients [1], astrocytic TRPV1 activation by CAP or related compounds might constitute a new therapeutic strategy to treat PD.
Figures
References
- Nam JH, Park ES, Won SY, Lee YA, Kim KI, Jeong JY, Baek JY, Cho EJ, Jin M, Chung YC, Lee BD, Kim SH, Kim EG, Byun K, Lee B, Woo DH, Lee CJ, Kim SR, Bok E, Kim YS, Ahn TB, Ko HW, Brahmachari S, Pletinkova O, Troconso JC, Dawson VL, Dawson TM, Jin BK. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson's disease via CNTF. Brain 2015;138:3610-3622.
- Won SY, Park MH, You ST, Choi SW, Kim HK, McLean C, Bae SC, Kim SR, Jin BK, Lee KH, Shin EY, Kim EG. Nigral dopaminergic PAK4 prevents neurodegeneration in rat models of Parkinson's disease. Sci Transl Med 2016;8:367ra170.
- Chung YC, Baek JY, Kim SR, Ko HW, Bok E, Shin WH, Won SY, Jin BK. Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson's disease. Exp Mol Med 2017;49:e298.
- Kong WL, Peng YY, Peng BW. Modulation of neuroinflammation: role and therapeutic potential of TRPV1 in the neuro-immune axis. Brain Behav Immun 2017;64:354-366.
- Park ES, Kim SR, Jin BK. Transient receptor potential vanilloid subtype 1 contributes to mesencephalic dopaminergic neuronal survival by inhibiting microglia-originated oxidative stress. Brain Res Bull 2012;89:92-96.
- Zhao Z, Wang J, Wang L, Yao X, Liu Y, Li Y, Chen S, Yue T, Wang X, Yu W, Liu Y. Capsaicin protects against oxidative insults and alleviates behavioral deficits in rats with 6-OHDA-induced Parkinson's disease via activation of TRPV1. Neurochem Res 2017;42:3431-3438.
- Hagg T, Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci U S A 1993;90:6315-6319.
- Jeong KH, Nam JH, Jin BK, Kim SR. Activation of CNTF/CNTFRα signaling pathway by hRheb(S16H) transduction of dopaminergic neurons in vivo. PLoS One 2015;10:e0121803.
- Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Disruption of the CNTF gene results in motor neuron degeneration. Nature 1993;365:27-32.
- Oyesiku NM, Wigston DJ. Ciliary neurotrophic factor stimulates neurite outgrowth from spinal cord neurons. J Comp Neurol 1996;364:68-77.
- Pease ME, Zack DJ, Berlinicke C, Bloom K, Cone F, Wang Y, Klein RL, Hauswirth WW, Quigley HA. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci 2009;50:2194-2200.
- Mittoux V, Joseph JM, Conde F, Palfi S, Dautry C, Poyot T, Bloch J, Deglon N, Ouary S, Nimchinsky EA, Brouillet E, Hof PR, Peschanski M, Aebischer P, Hantraye P. Restoration of cognitive and motor functions by ciliary neurotrophic factor in a primate model of Huntington's disease. Hum Gene Ther 2000;11:1177-1187.
- Chaturvedi RK, Agrawal AK, Seth K, Shukla S, Chauhan S, Shukla Y, Sinha C, Seth PK. Effect of glial cell line-derived neurotrophic factor (GDNF) co-transplantation with fetal ventral mesencephalic cells (VMC) on functional restoration in 6-hydroxydopamine (6-OHDA) lesioned rat model of Parkinson's disease: neurobehavioral, neurochemical and immunohistochemical studies. Int J Dev Neurosci 2003;21:391-400.
- Lindholm P, Voutilainen MH, Laurén J, Peränen J, Leppänen VM, Andressoo JO, Lindahl M, Janhunen S, Kalkkinen N, Timmusk T, Tuominen RK, Saarma M. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 2007;448:73-77.
- Yurek DM, Lu W, Hipkens S, Wiegand SJ. BDNF enhances the functional reinnervation of the striatum by grafted fetal dopamine neurons. Exp Neurol 1996;137:105-118.
- Gerhardt GA, Cass WA, Huettl P, Brock S, Zhang Z, Gash DM. GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res 1999;817:163-171.
- Kells AP, Eberling J, Su X, Pivirotto P, Bringas J, Hadaczek P, Narrow WC, Bowers WJ, Federoff HJ, Forsayeth J, Bankiewicz KS. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J Neurosci 2010;30:9567-9577.
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press, 2013.
- Veldhuis WB, van der Stelt M, Wadman MW, van Zadelhoff G, Maccarrone M, Fezza F, Veldink GA, Vliegenthart JF, Bär PR, Nicolay K, Di Marzo V. Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases. J Neurosci 2003;23:4127-4133.
- Jin BK, Iacovitti L. Dopamine differentiation factors increase striatal dopaminergic function in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mice. J Neurosci Res 1996;43:331-334.
- Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J, Johnson EM, Olanow CW, Mufson EJ, Kordower JH. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson's disease and nonhuman primate brains. Mov Disord 2011;26:27-36.
- Choi SH, Joe EH, Kim SU, Jin BK. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J Neurosci 2003;23:5877-5886.
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec 1991;231:482-497.
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science 2010;330:790-796.
- Shi X, Woodward WR, Habecker BA. Ciliary neurotrophic factor stimulates tyrosine hydroxylase activity. J Neurochem 2012;121:700-704.
- Shin WH, Jeon MT, Leem E, Won SY, Jeong KH, Park SJ, McLean C, Lee SJ, Jin BK, Jung UJ, Kim SR. Induction of microglial toll-like receptor 4 by prothrombin kringle-2: a potential pathogenic mechanism in Parkinson's disease. Sci Rep 2015;5:14764.
- Lapchak PA, Araujo DM, Hilt DC, Sheng J, Jiao S. Adenoviral vector-mediated GDNF gene therapy in a rodent lesion model of late stage Parkinson's disease. Brain Res 1997;777:153-160.
- Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Hanazono Y, Kume A, Urano F, Ichinose H, Nagatsu T, Nakano I, Ozawa K. Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson's disease. Gene Ther 2002;9:381-389.
- Wang L, Wang Z, Xu X, Zhu R, Bi J, Liu W, Feng X, Wu H, Zhang H, Wu J, Kong W, Yu B, Yu X. Recombinant AAV8-mediated intrastriatal gene delivery of CDNF protects rats against methamphetamine neurotoxicity. Int J Med Sci 2017;14:340-347.
- Huotarinen A, Penttinen AM, Bäck S, Voutilainen MH, Julku U, Piepponen TP, Männistö PT, Saarma M, Tuominen R, Laakso A, Airavaara M. Combination of CDNF and deep brain stimulation decreases neurological deficits in late-stage model Parkinson's disease. Neuroscience 2018;374:250-263.
- Jorge-Finnigan A, Kleppe R, Jung-Kc K, Ying M, Marie M, Rios-Mondragon I, Salvatore MF, Saraste J, Martinez A. Phosphorylation at serine 31 targets tyrosine hydroxylase to vesicles for transport along microtubules. J Biol Chem 2017;292:14092-14107.
- Herman JP, Rouge-Pont F, Le Moal M, Abrous DN. Mechanisms of amphetamine-induced rotation in rats with unilateral intrastriatal grafts of embryonic dopaminergic neurons: a pharmacological and biochemical analysis. Neuroscience 1993;53:1083-1095.
- Salvatore MF. Ser31 tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J Neurochem 2014;129:548-558.
- Baek JY, Jeong JY, Kim KI, Won SY, Chung YC, Nam JH, Cho EJ, Ahn TB, Bok E, Shin WH, Jin BK. Inhibition of microglia-derived oxidative stress by ciliary neurotrophic factor protects dopamine neurons in vivo from MPP+ neurotoxicity. Int J Mol Sci 2018;19:E3543.
- Reiness CG, Seppa MJ, Dion DM, Sweeney S, Foster DN, Nishi R. Chick ciliary neurotrophic factor is secreted via a nonclassical pathway. Mol Cell Neurosci 2001;17:931-944.
- Stöckli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Götz R, Lindholm D, Thoenen H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature 1989;342:920-923.
- Lin LF, Mismer D, Lile JD, Armes LG, Butler ET, Vannice JL, Collins F. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF). Science 1989;246:1023-1025.
- Kamiguchi H, Yoshida K, Sagoh M, Sasaki H, Inaba M, Wakamoto H, Otani M, Toya S. Release of ciliary neurotrophic factor from cultured astrocytes and its modulation by cytokines. Neurochem Res 1995;20:1187-1193.



